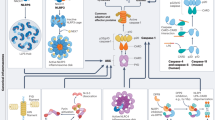
Abstract
Recent studies indicate that the human lipopolysaccharide sensor caspase-4, unlike its mouse homologue caspase-11, is constitutively expressed and activates pro-IL-18 as well as gasdermin D-mediated pyroptosis. Activation of human caspase-4 causes vascular leakage systemically and at the blood–brain barrier in mice and is implicated in the pathogenesis of a range of inflammatory diseases for which there are currently no effective therapies. These results suggest the therapeutic potential of modulating caspase-4 activity, and structural studies indicate that the caspase-4 exosite might be a promising inhibitory target.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ross, C. et al. Inflammatory caspases: toward a unified model for caspase activation by inflammasomes. Annu. Rev. Immunol. 40, 1–21 (2022).
Coll, R. C. & Schroder, K. Inflammasome components as new therapeutic targets in inflammatory disease. Nat. Rev. Immunol. 25, 22–41 (2024).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Fu, J., Schroder, K. & Wu, H. Mechanistic insights from inflammasome structures. Nat. Rev. Immunol. 24, 518–535 (2024).
Boucher, D. et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J. Exp. Med. 215, 827–840 (2018).
Ross, C., Chan, A. H., Pein, J. V., Boucher, D. & Schroder, K. Dimerization and auto-processing induce caspase-11 protease activation within the non-canonical inflammasome. Life Sci. Alliance 1, e201800237 (2018).
Lee, B. L. et al. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J. Exp. Med. 215, 2279–2288 (2018).
Chan, A. H. et al. Caspase-4 dimerisation and D289 auto-processing elicit an interleukin-1β-converting enzyme. Life Sci. Alliance 6, e202301908 (2023).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Zanoni, I. et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236 (2016).
Thornberry, N. A. et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. J. Biol. Chem. 272, 17907–17911 (1997).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Liu, X., Xia, S., Zhang, Z., Wu, H. & Lieberman, J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug. Discov. 20, 384–405 (2021).
Miao, R. et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity 56, 2523–2541 (2023).
Zanoni, I., Tan, Y., Gioia, M. D., Springstead, J. R. & Kagan, J. C. By capturing inflammatory lipids released from dying cells, the receptor CD14 induces inflammasome-dependent phagocyte hyperactivation. Immunity 47, 697–709 (2017).
Zheng, D., Liwinski, T. & Elinav, E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 6, 36 (2020).
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021).
Wu, J., Fernandes-Alnemri, T. & Alnemri, E. S. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J. Clin. Immunol. 30, 693–702 (2010).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1 activating inflammasome with ASC. Nature 458, 514–518 (2009).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Rathinam, V. A. K., Zhao, Y. & Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 20, 527–533 (2019).
Akuma, D. C. et al. Catalytic activity and autoprocessing of murine caspase-11 mediate noncanonical inflammasome assembly in response to cytosolic LPS. eLife 13, e83725 (2024).
Sharif, H. et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570, 338–343 (2019).
Andreeva, L. et al. NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell 184, 6299–6312 (2021).
Hollingsworth, L. R. et al. DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature 592, 778–783 (2021).
Paidimuddala, B. et al. Mechanism of NAIP—NLRC4 inflammasome activation revealed by cryo-EM structure of unliganded NAIP5. Nat. Struct. Mol. Biol. 30, 159–166 (2023).
Zhang, Z. et al. Structural basis for thioredoxin-mediated suppression of NLRP1 inflammasome. Nature 622, 188–194 (2023).
Xiao, L., Magupalli, V. G. & Wu, H. Cryo-EM structures of the active NLRP3 inflammasome disc. Nature 613, 595–600 (2023).
Vanaja, S. K. et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 165, 1106–1119 (2016).
Santos, J. C. et al. LPS targets host guanylate‐binding proteins to the bacterial outer membrane for non‐canonical inflammasome activation. EMBO J. 37, e98089 (2018).
Wandel, M. P. et al. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat. Immunol. 21, 880–891 (2020).
Santos, J. C. et al. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 11, 3276 (2020).
Zhu, S. et al. Native architecture of a human GBP1 defense complex for cell-autonomous immunity to infection. Science 383, eabm9903 (2024).
Kuhm, T. et al. Structural basis of antimicrobial membrane coat assembly by human GBP1. Nat. Struct. Mol. Biol. 32, 172–184. (2024).
Hara, H. et al. The NLRP6 inflammasome recognizes lipoteichoic acid and regulates Gram-positive pathogen infection. Cell 175, 1651–1664 (2018).
Chaves, M. M. et al. Non-canonical NLRP3 inflammasome activation and IL-1β signaling are necessary to L. amazonensis control mediated by P2X7 receptor and leukotriene B4. PLoS Pathog. 15, e1007887 (2019).
de Carvalho, R. V. H. et al. Leishmania lipophosphoglycan triggers caspase-11 and the non-canonical activation of the NLRP3 inflammasome. Cell Rep. 26, 429–437 (2019).
Celias, D. P. et al. Cathepsin L3 from fasciola hepatica induces NLRP3 inflammasome alternative activation in murine dendritic cells. Front. Immunol. 10, 552 (2019).
Kang, R. et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 24, 97–108 (2018).
Xu, W. et al. Apaf-1 pyroptosome senses mitochondrial permeability transition. Cell Metab. 33, 424–436 (2021).
Chukai, Y., Ito, G., Konno, M., Sakata, Y. & Ozaki, T. Mitochondrial calpain-5 truncates caspase-4 during endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 608, 156–162 (2022).
Chu, L. H. et al. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat. Commun. 9, 996 (2018).
Zhivaki, D. & Kagan, J. C. Innate immune detection of lipid oxidation as a threat assessment strategy. Nat. Rev. Immunol. 22, 322–330 (2022).
Rühl, S. et al. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960 (2018).
Schmid‐Burgk, J. L. et al. Caspase‐4 mediates non‐canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 45, 2911–2917 (2015).
Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Fernandes-Alnemri, T. et al. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J. Immunol. 191, 3995–3999 (2013).
Benaoudia, S. et al. A genome‐wide screen identifies IRF2 as a key regulator of caspase‐4 in human cells. EMBO Rep. 20, e48235 (2019).
Kayagaki, N. et al. IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci. Signal. 12, eaax4917 (2019).
Shi, X. et al. Recognition and maturation of IL-18 by caspase-4 noncanonical inflammasome. Nature 624, 442–450 (2023).
Eckhart, L. et al. Identification of a novel exon encoding the amino-terminus of the predominant caspase-5 variants. Biochem. Biophys. Res. Commun. 348, 682–688 (2006).
Lin, X. Y., Choi, M. S. K. & Porter, A. G. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-γ. J. Biol. Chem. 275, 39920–39926 (2000).
Kajiwara, Y. et al. A critical role for human caspase-4 in endotoxin sensitivity. J. Immunol. 193, 335–343 (2014).
Wei, C. et al. Brain endothelial GSDMD activation mediates inflammatory BBB breakdown. Nature 629, 893–900 (2024).
Lagrange, B. et al. Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11. Nat. Commun. 9, 242 (2018).
Warren, H. S. et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. J. Infect. Dis. 201, 223–232 (2010).
Marshall, J. C. Why have clinical trials in sepsis failed? Trends Mol. Med. 20, 195–203 (2014).
Conner, J. R., Smirnova, I. I. & Poltorak, A. A mutation in Irak2c identifies IRAK-2 as a central component of the TLR regulatory network of wild-derived mice. J. Exp. Med. 206, 1615–1631 (2009).
Schroder, K. et al. Conservation and divergence in Toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages. Proc. Natl Acad. Sci. 109, E944–E953 (2012).
Rojas-Lopez, M. et al. NLRP11 is a pattern recognition receptor for bacterial lipopolysaccharide in the cytosol of human macrophages. Sci. Immunol. 8, eabo4767 (2023).
Ghayur, T. et al. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN- γ production. Nature 386, 619–623 (1997).
Margolin, N. et al. Substrate and inhibitor specificity of interleukin-1β-converting enzyme and related caspases. J. Biol. Chem. 272, 7223–7228 (1997).
Knodler, L. A. et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014).
Bibo-Verdugo, B., Snipas, S. J., Kolt, S., Poreba, M. & Salvesen, G. S. Extended subsite profiling of the pyroptosis effector protein gasdermin D reveals a region recognized by inflammatory caspase-11. J. Biol. Chem. 295, 11292–11302 (2020).
Devant, P. et al. Structural insights into cytokine cleavage by inflammatory caspase-4. Nature 624, 451–459 (2023).
Exconde, P. M. et al. The tetrapeptide sequence of IL-18 and IL-1β regulates their recruitment and activation by inflammatory caspases. Cell Rep. 42, 113581 (2023).
Li, Z. et al. Shigella evades pyroptosis by arginine ADP-riboxanation of caspase-11. Nature 599, 290–295 (2021).
Wang, K. et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell 180, 941–955 (2020).
Cheng, K. T. et al. Caspase-11–mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J. Clin. Invest. 127, 4124–4135 (2017).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Napier, B. A. et al. Complement pathway amplifies caspase-11–dependent cell death and endotoxin-induced sepsis severity. J. Exp. Med. 213, 2365–2382 (2016).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Gyawali, B., Ramakrishna, K. & Dhamoon, A. S. Sepsis: the evolution in definition, pathophysiology, and management. SAGE Open Med. 7, 2050312119835043 (2019).
Wang, M., Feng, J., Zhou, D. & Wang, J. Bacterial lipopolysaccharide-induced endothelial activation and dysfunction: a new predictive and therapeutic paradigm for sepsis. Eur. J. Méd. Res. 28, 339 (2023).
Meurens, F., Summerfield, A., Nauwynck, H., Saif, L. & Gerdts, V. The pig: a model for human infectious diseases. Trends Microbiol. 20, 50–57 (2012).
Rutai, A. et al. A porcine sepsis model with numerical scoring for early prediction of severity. Front. Med. 9, 867796 (2022).
Amali, A. A. et al. Extracorporeal membrane oxygenation–dependent fulminant melioidosis from caspase 4 mutation reversed by interferon γ therapy. Clin. Infect. Dis. 78, 94–97 (2023).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Jorgensen, I., Zhang, Y., Krantz, B. A. & Miao, E. A. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med. 213, 2113–2128 (2016).
Guo, Q. et al. Organism type of infection is associated with prognosis in sepsis: an analysis from the MIMIC-IV database. BMC Infect. Dis. 23, 431 (2023).
Xu, G. et al. Gasdermin D protects against Streptococcus equi subsp. zooepidemicus infection through macrophage pyroptosis. Front. Immunol. 13, 1005925 (2022).
Eltobgy, M. M. et al. Caspase-4/11 exacerbates disease severity in SARS–CoV-2 infection by promoting inflammation and immunothrombosis. Proc. Natl Acad. Sci. 119, e2202012119 (2022).
Wu, Y. et al. Caspase-4/11–mediated pulmonary artery endothelial cell pyroptosis contributes to pulmonary arterial hypertension. Hypertension 79, 536–548 (2022).
Zasłona, Z. et al. Caspase-11 promotes allergic airway inflammation. Nat. Commun. 11, 1055 (2020).
Flood, B. et al. Altered expression of caspases‐4 and ‐5 during inflammatory bowel disease and colorectal cancer: diagnostic and therapeutic potential. Clin. Exp. Immunol. 181, 39–50 (2015).
Kanai, T. et al. Interleukin 18 is a potent proliferative factor for intestinal mucosal lymphocytes in Crohn’s disease. Gastroenterology 119, 1514–1523 (2000).
Haep, L. et al. Interferon gamma counteracts the angiogenic switch and induces vascular permeability in dextran sulfate sodium colitis in mice. Inflamm. Bowel Dis. 21, 2360–2371 (2015).
Naseer, N. et al. Salmonella enterica serovar Typhimurium induces NAIP/NLRC4- and NLRP3/ASC-independent, caspase-4-dependent inflammasome activation in human intestinal epithelial cells. Infect. Immun. 90, e00663–21 (2022).
Shi, H. et al. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ. Res. 129, 383–396 (2021).
Yanpiset, P. et al. Gasdermin D-mediated pyroptosis in myocardial ischemia and reperfusion injury: cumulative evidence for future cardioprotective strategies. Acta Pharm. Sin. B 13, 29–53 (2023).
Hisahara, S., Yuan, J., Momoi, T., Okano, H. & Miura, M. Caspase-11 mediates oligodendrocyte cell death and pathogenesis of autoimmune-mediated demyelination. J. Exp. Med. 193, 111–122 (2001).
Kang, S. J., Sanchez, I., Jing, N. & Yuan, J. Dissociation between neurodegeneration and caspase-11-mediated activation of caspase-1 and caspase-3 in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 23, 5455–5460 (2003).
Yin, P. et al. Caspase-4 mediates cytoplasmic accumulation of TDP-43 in the primate brains. Acta Neuropathol. 137, 919–937 (2019).
Landy, E., Carol, H., Ring, A. & Canna, S. Biological and clinical roles of IL-18 in inflammatory diseases. Nat. Rev. Rheumatol. 20, 33–47 (2024).
Moran, B. et al. Targeting the NLRP3 inflammasome reduces inflammation in hidradenitis suppurativa skin. Br. J. Dermatol. 189, 447–458 (2023).
Degterev, A., Boyce, M. & Yuan, J. A decade of caspases. Oncogene 22, 8543–8567 (2003).
Wannamaker, W. et al. (S)-1-((S)-2-{[1-(4-Amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1β and IL-18. J. Pharmacol. Exp. Ther. 321, 509–516 (2007).
Méthot, N. et al. A caspase active site probe reveals high fractional inhibition needed to block DNA fragmentation. J. Biol. Chem. 279, 27905–27914 (2004).
Méthot, N. et al. Differential efficacy of caspase inhibitors on apoptosis markers during sepsis in rats and implication for fractional inhibition requirements for therapeutics. J. Exp. Med. 199, 199–207 (2004).
McStay, G. P., Salvesen, G. S. & Green, D. R. Overlapping cleavage motif selectivity of caspases: implications for analysis of apoptotic pathways. Cell Death Differ. 15, 322–331 (2008).
Hardy, J. A. & Wells, J. A. Searching for new allosteric sites in enzymes. Curr. Opin. Struct. Biol. 14, 706–715 (2004).
Hardy, J. A., Lam, J., Nguyen, J. T., O’Brien, T. & Wells, J. A. Discovery of an allosteric site in the caspases. Proc. Natl Acad. Sci. USA 101, 12461–12466 (2004).
Scheer, J. M., Romanowski, M. J. & Wells, J. A. A common allosteric site and mechanism in caspases. Proc. Natl Acad. Sci. USA 103, 7595–7600 (2006).
Wang, X. et al. A small molecule binding HMGB1 inhibits caspase-11-mediated lethality in sepsis. Cell Death Dis. 12, 402 (2021).
Sun, W. et al. Beclin1 controls caspase-4 inflammsome activation and pyroptosis in mouse myocardial reperfusion-induced microvascular injury. Cell Commun. Signal. 19, 107 (2021).
Alrumayyan, N. et al. Prolidase deficiency, a rare inborn error of immunity, clinical phenotypes, immunological features, and proposed treatments in twins. Allergy Asthma Clin. Immunol. 18, 17 (2022).
Coppola, S. et al. Mutations at the C-terminus of CDC42 cause distinct hematopoietic and autoinflammatory disorders. J. Allergy Clin. Immunol. 150, 223–228 (2022).
Liu, Z. et al. Crystal structures of the full-length murine and human gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity 51, 43–49 (2019).
Acknowledgements
This work was supported in part by National Institutes of Health (NIH) grants CA240955 (to J.L.), and AI182369 and CA287076 (to H.W. and J.L.).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
All the authors are associated with Ventus Therapeutics (E.E., F.G.G. and M.A.C. as employees; H.W. and J.L. as cofounders and advisers) and have stock options.
Peer review
Peer review information
Nature Reviews Immunology thanks Dave Boucher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elkayam, E., Gervais, F.G., Wu, H. et al. New insights into the noncanonical inflammasome point to caspase-4 as a druggable target. Nat Rev Immunol 25, 558–568 (2025). https://doi.org/10.1038/s41577-025-01142-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-025-01142-9