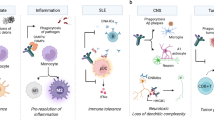
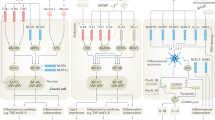
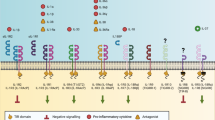
Abstract
Many inhibitory receptors that regulate immune cell function recognize a limited number of specific ligands. However, a subgroup of so-called inhibitory pattern recognition receptors (iPRRs) can bind a much larger array of ligands of structural similarity. Leukocyte-associated immunoglobulin-like receptor 1 (LAIR1) is one such iPRR that is expressed by most immune cells and recognizes a common structural pattern present in collagens and collagen domain-containing proteins. LAIR1 signalling regulates diverse immune cell populations and is currently the focus of multiple clinical trials for the treatment of cancer. We here review the current literature on LAIR1, as a prototypic example of how inhibitory PRRs contribute to immune balance and of how these receptors are regulated. We discuss the function of LAIR1 in homeostasis, infection, inflammation and cancer, and consider the advantages and potential pitfalls of targeting this receptor in human disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rumpret, M. et al. Functional categories of immune inhibitory receptors. Nat. Rev. Immunol. 20, 771–780 (2020).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Grebinoski, S. & Vignali, D. A. Inhibitory receptor agonists: the future of autoimmune disease therapeutics? Curr. Opin. Immunol. 67, 1–9 (2020).
Singh, A. et al. A novel bioinformatics pipeline for the identification of immune inhibitory receptors as potential therapeutic targets. eLife 13, RP92870 (2024).
Rumpret, M., von Richthofen, H. J., Peperzak, V. & Meyaard, L. Inhibitory pattern recognition receptors. J. Exp. Med. 219, e20211463 (2022).
Kumawat, K. et al. LAIR-1 limits neutrophilic airway inflammation. Front. Immunol. 10, 842 (2019).
Helou, D. G. et al. LAIR-1 acts as an immune checkpoint on activated ILC2s and regulates the induction of airway hyperreactivity. J. Allergy Clin. Immunol. 149, 223–236.e6 (2022). This paper uses mouse in vivo assays, signalling read-outs and ex vivo human cells to study how LAIR1 regulates type 2 innate lymphoid cells in airway hyperreactivity.
Carvalheiro, T. et al. Impaired LAIR-1-mediated immune control due to collagen degradation in fibrosis. J. Autoimmun. 146, 103219 (2024).
Kim, S. et al. The role of leukocyte-associated Ig-like receptor-1 in suppressing collagen-induced arthritis. J. Immunol. 199, 2692–2700 (2017).
Myers, L. K. et al. 1,25-Dihydroxyvitamin D3 and 20-hydroxyvitamin D3 upregulate LAIR-1 and attenuate collagen induced arthritis. Int. J. Mol. Sci. 22, 13342 (2021).
Carroll, K. R. et al. Lupus autoantibodies initiate neuroinflammation sustained by continuous HMGB1:RAGE signaling and reversed by increased LAIR-1 expression. Nat. Immunol. 25, 671–681 (2024). This paper emphasizes HMGB1–RAGE and C1q–LAIR-1 interactions as key pathways in the microglial–neuronal interplay that determines the balance between physiological and maladaptive states.
Camargo, C. M., Augusto, D. G. & Petzl-Erler, M. L. Differential gene expression levels might explain association of LAIR2 polymorphisms with pemphigus. Hum. Genet. 135, 233–244 (2016).
Farias, T. D. J., Augusto, D. G., de Almeida, R. C., Malheiros, D. & Petzl-Erler, M. L. Screening the full leucocyte receptor complex genomic region revealed associations with pemphigus that might be explained by gene regulation. Immunology 156, 86–93 (2019).
Diaz-Pena, R. et al. A high density SNP genotyping approach within the 19q13 chromosome region identifies an association of a CNOT3 polymorphism with ankylosing spondylitis. Ann. Rheum. Dis. 71, 714–717 (2012).
Simone, R. et al. Serum LAIR-2 is increased in autoimmune thyroid diseases. PLoS One 8, e63282 (2013).
Elsayed, O. M., Abdelazim, S. A., Darwish, H. A., Shaker, O. G. & Senousy, M. A. Association of LncRNA–PAX8–AS1 and LAIR-2 polymorphisms along with their expression with clinical and subclinical hypothyroidism. Sci. Rep. 13, 6 (2023).
Flies, D. B., Langermann, S., Jensen, C., Karsdal, M. A. & Willumsen, N. Regulation of tumor immunity and immunotherapy by the tumor collagen extracellular matrix. Front. Immunol. 14, 1199513 (2023).
Chang, Y. C. & Nizet, V. The interplay between Siglecs and sialylated pathogens. Glycobiology 24, 818–825 (2014).
Yang, T. et al. LILRB4, an immune checkpoint on myeloid cells. Blood Sci. 4, 49–56 (2022).
Wang, Y. et al. Discovery of galectin-8 as an LILRB4 ligand driving M-MDSCs defines a class of antibodies to fight solid tumors. Cell Rep. Med. 5, 101374 (2024).
Meyaard, L. et al. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 7, 283–290 (1997).
Wende, H., Volz, A. & Ziegler, A. Extensive gene duplications and a large inversion characterize the human leukocyte receptor cluster. Immunogenetics 51, 703–713 (2000).
Lebbink, R. J. et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 203, 1419–1425 (2006).
Lebbink, R. J. et al. Identification of multiple potent binding sites for human leukocyte associated Ig-like receptor LAIR on collagens II and III. Matrix Biol. 28, 202–210 (2009).
Son, M., Santiago-Schwarz, F., Al-Abed, Y. & Diamond, B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc. Natl Acad. Sci. USA 109, E3160–E3167 (2012).
Olde Nordkamp, M. J. et al. Inhibition of the classical and lectin pathway of the complement system by recombinant LAIR-2. J. Innate Immun. 6, 284–292 (2014).
Olde Nordkamp, M. J. et al. Leukocyte-associated Ig-like receptor-1 is a novel inhibitory receptor for surfactant protein D. J. Leukoc. Biol. 96, 105–111 (2014).
Keerthivasan, S. et al. Homeostatic functions of monocytes and interstitial lung macrophages are regulated via collagen domain-binding receptor LAIR1. Immunity 54, 1511–1526.e8 (2021). This work identifies a new ligand for LAIR1 and shows that LAIR1 regulates homeostatic pathways in macrophages, which affects tumour immunity.
Zhang, Y. S. et al. Adiponectin’s globular domain inhibits T cell activation by interacting with LAIR-1. Biochem. Biophys. Res. Commun. 573, 117–124 (2021).
Tang, X. B. et al. A single residue, arginine 65, is critical for the functional interaction of leukocyte-associated inhibitory receptor-1 with collagens. J. Immunol. 182, 5446–5452 (2009).
Fouet, G. et al. Molecular basis of complement C1q collagen-like region interaction with the immunoglobulin-like receptor LAIR-1. Int. J. Mol. Sci. 22, 5125 (2021).
Meyaard, L., Hurenkamp, J., Clevers, H., Lanier, L. L. & Phillips, J. H. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J. Immunol. 162, 5800–5804 (1999).
van der Vuurst de Vries, A. R., Clevers, H., Logtenberg, T. & Meyaard, L. Leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) is differentially expressed during human B cell differentiation and inhibits B cell receptor-mediated signaling. Eur. J. Immunol. 29, 3160–3167 (1999).
Merlo, A. et al. Inhibitory receptors CD85j, LAIR-1, and CD152 down-regulate immunoglobulin and cytokine production by human B lymphocytes. Clin. Diagn. Lab. Immunol. 12, 705–712 (2005).
Fournier, N. et al. FDF03, a novel inhibitory receptor of the immunoglobulin superfamily, is expressed by human dendritic and myeloid cells. J. Immunol. 165, 1197–1209 (2000).
Verbrugge, A., Ruiter Td, T., Clevers, H. & Meyaard, L. Differential contribution of the immunoreceptor tyrosine-based inhibitory motifs of human leukocyte-associated Ig-like receptor-1 to inhibitory function and phosphatase recruitment. Int. Immunol. 15, 1349–1358 (2003).
Tomlinson, M. G. et al. Collagen promotes sustained GPVI signaling in platelets and cell lines. J. Thromb. Haemost. 5, 2274–2283 (2007).
Carvalheiro, T. et al. Leukocyte associated immunoglobulin like receptor 1 regulation and function on monocytes and dendritic cells during inflammation. Front. Immunol. 11, 1793 (2020).
Bonaccorsi, I. et al. The immune inhibitory receptor LAIR-1 is highly expressed by plasmacytoid dendritic cells and acts complementary with NKp44 to control IFNɑ production. PLoS One 5, e15080 (2010).
Zocchi, M. R., Pellegatta, F., Pierri, I., Gobbi, M. & Poggi, A. Leukocyte-associated Ig-like receptor-1 prevents granulocyte–monocyte colony stimulating factor-dependent proliferation and Akt1/PKBɑ activation in primary acute myeloid leukemia cells. Eur. J. Immunol. 31, 3667–3675 (2001).
Xue, J. et al. Leukocyte-associated immunoglobulin-like receptor-1 is expressed on human megakaryocytes and negatively regulates the maturation of primary megakaryocytic progenitors and cell line. Biochem. Biophys. Res. Commun. 405, 128–133 (2011).
Fu, Q. et al. Involvement of the JAK–STAT pathway in collagen regulation of decidual NK cells. Am. J. Reprod. Immunol. https://doi.org/10.1111/aji.12769 (2017).
Maasho, K. et al. The inhibitory leukocyte-associated Ig-like receptor-1 (LAIR-1) is expressed at high levels by human naive T cells and inhibits TCR mediated activation. Mol. Immunol. 42, 1521–1530 (2005).
Singh, A. et al. Leukocyte-associated immunoglobulin-like receptor-1 blockade in combination with programmed death-ligand 1 targeting therapy mediates increased tumour control in mice. Cancer Immunol. Immunother. 73, 16 (2024).
Agashe, V. V. et al. Leukocyte-associated Ig-like receptor 1 inhibits TH1 responses but is required for natural and induced monocyte-dependent TH17 responses. J. Immunol. 201, 772–781 (2018).
Ravetch, J. V. & Lanier, L. L. Immune inhibitory receptors. Science 290, 84–89 (2000).
Verbrugge, A., Rijkers, E. S. K., De Ruiter, T. & Meyaard, L. Leukocyte-associated Ig-like receptor-1 has SH-2 domain-containing phosphatase-independent function and recruits C-terminal Src kinase. Eur. J. Immunol. 36, 190–198 (2006).
Park, J. E. et al. Leukocyte-associated immunoglobulin-like receptor 1 inhibits T-cell signaling by decreasing protein phosphorylation in the T-cell signaling pathway. J. Biol. Chem. 295, 2239–2247 (2020).
Sayos, J., Martinez-Barriocanal, A., Kitzig, F., Bellon, T. & Lopez-Botet, M. Recruitment of C-terminal Src kinase by the leukocyte inhibitory receptor CD85j. Biochem. Biophys. Res. Commun. 324, 640–647 (2004).
Veillette, A., Thibaudeau, E. & Latour, S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem. 273, 22719–22728 (1998).
Tourdot, B. E. et al. Immunoreceptor tyrosine-based inhibitory motif (ITIM)-mediated inhibitory signaling is regulated by sequential phosphorylation mediated by distinct nonreceptor tyrosine kinases: a case study involving PECAM-1. Biochemistry 52, 2597–2608 (2013).
Liu, H. et al. A comprehensive immunoreceptor phosphotyrosine-based signaling network revealed by reciprocal protein–peptide array screening. Mol. Cell Proteom. 14, 1846–1858 (2015).
Pfrepper, K. I. et al. Structural and functional dissection of the cytoplasmic domain of the transmembrane adaptor protein SIT (SHP2-interacting transmembrane adaptor protein). Eur. J. Immunol. 31, 1825–1836 (2001).
Montgomery, B. C., Cortes, H. D., Burshtyn, D. N. & Stafford, J. L. Channel catfish leukocyte immune-type receptor mediated inhibition of cellular cytotoxicity is facilitated by SHP-1-dependent and -independent mechanisms. Dev. Comp. Immunol. 37, 151–163 (2012).
Nada, S., Okada, M., MacAuley, A., Cooper, J. A. & Nakagawa, H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature 351, 69–72 (1991).
Cloutier, J. F. & Veillette, A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J. Exp. Med. 189, 111–121 (1999).
Son, M. & Diamond, B. C1q-mediated repression of human monocytes is regulated by leukocyte-associated Ig-like receptor 1 (LAIR-1). Mol. Med. 20, 559–568 (2015).
Shi, L. et al. SIRPɑ sequesters SHP-2 to promote IL-4 and IL-13 signaling and the alternative activation of macrophages. Sci. Signal. 14, eabb3966 (2021).
Xu, M. J., Zhao, R. X. & Zhao, Z. Z. J. Identification and characterization of leukocyte-associated Ig-like receptor-1 as a major anchor protein of tyrosine phosphatase SHP-1 in hematopoietic cells. J. Biol. Chem. 275, 17440–17446 (2000).
Kogata, N. et al. Identification of Fer tyrosine kinase localized on microtubules as a platelet endothelial cell adhesion molecule-1 phosphorylating kinase in vascular endothelial cells. Mol. Biol. Cell 14, 3553–3564 (2003).
Sathish, J. G. et al. Constitutive association of SHP-1 with leukocyte-associated Ig-like receptor-1 in human T cells. J. Immunol. 166, 1763–1770 (2001).
Geerdink, R. J. et al. LAIR-1 limits neutrophil extracellular trap formation in viral bronchiolitis. J. Allergy Clin. Immunol. 141, 811–814 (2018).
Lominadze, G. et al. Proteomic analysis of human neutrophil granules. Mol. Cell Proteom. 4, 1503–1521 (2005).
Rorvig, S., Ostergaard, O., Heegaard, N. H. & Borregaard, N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. J. Leukoc. Biol. 94, 711–721 (2013).
Verbrugge, A., de Ruiter, T., Geest, C., Coffer, P. J. & Meyaard, L. Differential expression of leukocyte-associated Ig-like receptor-1 during neutrophil differentiation and activation. J. Leukoc. Biol. 79, 828–836 (2006).
Seery, V. et al. Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. EBioMedicine 67, 103357 (2021).
Cao, Q. et al. Five ETS family members, ELF-1, ETV-4, ETV-3L, ETS-1, and ETS-2 upregulate human leukocyte-associated immunoglobulin-like receptor-1 gene basic promoter activity. Aging 10, 1390–1401 (2018).
Dorando, H. K. et al. LPS and type I and II interferons have opposing effects on epigenetic regulation of LAIR1 expression in mouse and human macrophages. J. Leukoc. Biol. 115, 547–564 (2024).
Kim, W. M., Huang, Y. H., Gandhi, A. & Blumberg, R. S. CEACAM1 structure and function in immunity and its therapeutic implications. Semin. Immunol. 42, 101296 (2019).
Ohnishi, H. et al. Ectodomain shedding of SHPS-1 and its role in regulation of cell migration. J. Biol. Chem. 279, 27878–27887 (2004).
Londino, J. D., Gulick, D., Isenberg, J. S. & Mallampalli, R. K. Cleavage of signal regulatory protein alpha (SIRPɑ) enhances inflammatory signaling. J. Biol. Chem. 290, 31113–31125 (2015).
Eugenin, E. A. et al. Shedding of PECAM-1 during HIV infection: a potential role for soluble PECAM-1 in the pathogenesis of NeuroAIDS. J. Leukoc. Biol. 79, 444–452 (2006).
von Richthofen, H. J. et al. Soluble signal inhibitory receptor on leukocytes-1 is released from activated neutrophils by proteinase 3 cleavage. J. Immunol. 210, 389–397 (2023).
Ouyang, W. et al. Establishment of an ELISA system for determining soluble LAIR-1 levels in sera of patients with HFRS and kidney transplant. J. Immunol. Methods 292, 109–117 (2004).
Olde Nordkamp, M. J. M. et al. Enhanced secretion of leukocyte-associated immunoglobulin-like receptor (LAIR)-2 and soluble LAIR-1 in rheumatoid arthritis: LAIR-2 is a more efficient antagonist of the LAIR-1-collagen inhibitory interaction than soluble LAIR -1. Arthritis Rheum. 63, 3749–3757 (2011).
Lebbink, R. J. et al. The soluble leukocyte-associated Ig-like receptor (LAIR)-2 antagonizes the collagen/LAIR-1 inhibitory immune interaction. J. Immunol. 180, 1662–1669 (2008).
Xie, X. et al. Development and evaluation of a sandwich ELISA method for the detection of human CD306. J. Immunol. Methods 396, 65–73 (2013).
Wang, P. et al. Dissecting T-cell heterogeneity in esophageal squamous cell carcinoma reveals the potential role of LAIR2 in antitumor immunity. Clin. Exp. Immunol. 214, 36–49 (2023).
Pan, B. et al. TGFβ–p-STAT1–LAIR2 axis has a “self-rescue” role for exhausted CD8+ T cells in hepatocellular carcinoma. Cell Oncol. 46, 1625–1644 (2023).
Mehta, N. K. et al. CLN-617 retains IL2 and IL12 in injected tumors to drive robust and systemic immune-mediated antitumor activity. Cancer Immunol. Res. 12, 1022–1038 (2024).
Ramos, M. I. P. et al. Cancer immunotherapy by NC410, a LAIR-2 Fc protein blocking human LAIR-collagen interaction. eLife 10, e62927 (2021).
Founds, S. A., Fallert-Junecko, B., Reinhart, T. A., Conley, Y. P. & Parks, W. T. LAIR2 localizes specifically to sites of extravillous trophoblast invasion. Placenta 31, 880–885 (2010).
Fu, Q. et al. Human decidua mesenchymal stem cells regulate decidual natural killer cell function via interactions between collagen and leukocyte-associated immunoglobulin-like receptor 1. Mol. Med. Rep. 16, 2791–2798 (2017).
Cox, T. R. The matrix in cancer. Nat. Rev. Cancer 21, 217–238 (2021).
Borst, R., Meyaard, L. & Pascoal Ramos, M. I. Understanding the matrix: collagen modifications in tumors and their implications for immunotherapy. J. Transl. Med. 22, 382 (2024).
Rygiel, T. P., Stolte, E. H., de Ruiter, T., van de Weijer, M. L. & Meyaard, L. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol. Immunol. 49, 402–406 (2011).
Vijver, S. V. et al. Collagen fragments produced in cancer mediate T cell suppression through leukocyte-associated immunoglobulin-like receptor 1. Front. Immunol. 12, 733561 (2021).
Myers, L. K. et al. Role of citrullinated collagen in autoimmune arthritis. Int. J. Mol. Sci. 23, 9833 (2022).
Yu, X. et al. Reducing affinity as a strategy to boost immunomodulatory antibody agonism. Nature 614, 539–547 (2023). This work shows that reducing the affinity of antibodies for immune receptors can turn antagonists into agonists.
Muthana, S. M., Campbell, C. T. & Gildersleeve, J. C. Modifications of glycans: biological significance and therapeutic opportunities. ACS Chem. Biol. 7, 31–43 (2012).
Zhuo, Y., Yang, J. Y., Moremen, K. W. & Prestegard, J. H. Glycosylation alters dimerization properties of a cell-surface signaling protein, carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1). J. Biol. Chem. 291, 20085–20095 (2016).
Son, M. et al. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood 128, 2218–2228 (2016).
Son, M. et al. Evidence for C1q-mediated crosslinking of CD33/LAIR-1 inhibitory immunoreceptors and biological control of CD33/LAIR-1 expression. Sci. Rep. 7, 270 (2017).
Liu, T. et al. HMGB1–C1q complexes regulate macrophage function by switching between leukotriene and specialized proresolving mediator biosynthesis. Proc. Natl Acad. Sci. USA 116, 23254–23263 (2019).
Chen, Z. et al. Signalling thresholds and negative B-cell selection in acute lymphoblastic leukaemia. Nature 521, 357–361 (2015).
Poggi, A. et al. Lack of the leukocyte-associated Ig-like receptor-1 expression in high-risk chronic lymphocytic leukaemia results in the absence of a negative signal regulating kinase activation and cell division. Leukemia 22, 980–988 (2008).
Rawstron, A. C. et al. Chronic lymphocytic leukaemia (CLL) and CLL-type monoclonal B-cell lymphocytosis (MBL) show differential expression of molecules involved in lymphoid tissue homing. Cytom. B Clin. Cytom. 78, S42–S46 (2010).
Devin, J., Kassambara, A., Bruyer, A., Moreaux, J. & Bret, C. Phenotypic characterization of diffuse large B-cell lymphoma cells and prognostic impact. J. Clin. Med. 8, 1074 (2019).
Van Laethem, F. et al. LAIR1, an ITIM-containing receptor involved in immune disorders and in hematological neoplasms. Int. J. Mol. Sci. 23, 16136 (2022).
Andrews, J. M. et al. Novel cell adhesion/migration pathways are predictive markers of HDAC inhibitor resistance in cutaneous T cell lymphoma. EBioMedicine 46, 170–183 (2019).
Kang, X. et al. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat. Cell Biol. 17, 665–677 (2015). This work demonstrates that LAIR1, through the CAMK–CREB pathway, activates a survival mechanism essential for AML cell engraftment.
Lovewell, R. R. et al. LAIR-1 agonism as a therapy for acute myeloid leukemia. J. Clin. Invest. 133, e169519. (2023). This work demonstrates that agonistic antibodies targeting LAIR1 induce apoptosis specifically in AML cells, a response associated with their increased LAIR1 expression.
Peng, D. H. et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion. Nat. Commun. 11, 4520 (2020). This study identifies collagen-induced LAIR1 signalling as a key driver of T cell exhaustion and immunotherapy resistance, showing that targeting the collagen–LAIR1–SHP1 axis, via LOXL2 suppression, LAIR2 overexpression or SHP1 inhibition, enhances anti-PD1/PDL1 efficacy.
Joseph, C. et al. The ITIM-containing receptor: leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) modulates immune response and confers poor prognosis in invasive breast carcinoma. Cancers 13, 80 (2020).
Xu, L. J., Wang, S. L., Li, J. F., Li, J. & Li, B. Y. Cancer immunotherapy based on blocking immune suppression mediated by an immune modulator LAIR-1. Oncoimmunology 9, 1740477 (2020).
Wei, X. et al. LAIR1 drives glioma progression by nuclear focal adhesion kinase dependent expressions of cyclin D1 and immunosuppressive chemokines/cytokines. Cell Death Dis. 14, 684 (2023).
Chen, Z. et al. PNOC expressed by B cells in cholangiocarcinoma was survival related and LAIR2 could be a T cell exhaustion biomarker in tumor microenvironment: characterization of immune microenvironment combining single-cell and bulk sequencing technology. Front. Immunol. 12, 647209 (2021).
Chen, K. et al. Single-cell RNA-seq reveals dynamic change in tumor microenvironment during pancreatic ductal adenocarcinoma malignant progression. EBioMedicine 66, 103315 (2021).
Aung, T. N. et al. Quantitative, spatially defined expression of leukocyte-associated immunoglobulin-like receptor in non-small cell lung cancer. Cancer Res. Commun. 3, 471–482 (2023).
Jingushi, K. et al. Leukocyte-associated immunoglobulin-like receptor 1 promotes tumorigenesis in RCC. Oncol. Rep. 41, 1293–1303 (2019).
Pan, B. et al. LAIR1-mediated resistance of hepatocellular carcinoma cells to T cells through a GSK-3β/β-catenin/MYC/PD-L1 pathway. Cell Signal. 115, 111039 (2024).
Cao, Q. et al. Leukocyte-associated immunoglobulin-like receptor-1 expressed in epithelial ovarian cancer cells and involved in cell proliferation and invasion. Biochem. Biophys. Res. Commun. 458, 399–404 (2015).
Liu, Y. et al. LAIR-1 suppresses cell growth of ovarian cancer cell via the PI3K–AKT–mTOR pathway. Aging 12, 16142–16154 (2020).
Zhang, J. et al. LAIR-1 overexpression inhibits epithelial–mesenchymal transition in osteosarcoma via GLUT1-related energy metabolism. World J. Surg. Oncol. 18, 136 (2020).
Lenting, P. J., Westerlaken, G. H., Denis, C. V., Akkerman, J. W. & Meyaard, L. Efficient inhibition of collagen-induced platelet activation and adhesion by LAIR-2, a soluble Ig-like receptor family member. PLoS One 5, e12174 (2010).
Afshar-Kharghan, V. The role of the complement system in cancer. J. Clin. Invest. 127, 780–789 (2017).
Rogers, L. M., Veeramani, S. & Weiner, G. J. Complement in monoclonal antibody therapy of cancer. Immunol. Res. 59, 203–210 (2014).
Roumenina, L. T., Daugan, M. V., Petitprez, F., Sautes-Fridman, C. & Fridman, W. H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 19, 698–715 (2019).
Xie, J. et al. Blocking LAIR1 signaling in immune cells inhibits tumor development. Front. Immunol. 13, 996026 (2022).
Rodriguez, B. L. et al. Antitumor activity of a novel LAIR1 antagonist in combination with anti-PD1 to treat collagen-rich solid tumors. Mol. Cancer Ther. 23, 1144–1158 (2024).
Horn, L. A. et al. Remodeling the tumor microenvironment via blockade of LAIR-1 and TGFβ signaling enables PD-L1-mediated tumor eradication. J. Clin. Invest. https://doi.org/10.1172/JCI155148 (2022). This study shows that targeting collagen–LAIR1 signalling alongside TGFβ and PDL1 blockade remodels the tumour matrix, boosts CD8+ T cell responses, repolarizes macrophages and achieves durable cures in colon and mammary carcinoma models, underscoring the therapeutic value of combining ECM targeting with checkpoint inhibition.
Gibbons, A., Sundram, S. & Dean, B. Changes in non-coding RNA in depression and bipolar disorder: can they be used as diagnostic or theranostic biomarkers? Noncoding RNA 6, 33 (2020).
Haq, F., Ahmed, N. & Qasim, M. Comparative genomic analysis of collagen gene diversity. 3 Biotech 9, 83 (2019).
Elvington, M., Liszewski, M. K. & Atkinson, J. P. Evolution of the complement system: from defense of the single cell to guardian of the intravascular space. Immunol. Rev. 274, 9–15 (2016).
Lebbink, R. J. et al. Mouse LAIR-1 functions as an inhibitory collagen-binding receptor on immune cells. Int. Immunol. 19, 1011–1019 (2007).
Ali, N. et al. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rγnull mice display a T-effector memory phenotype. PLoS One 7, e44219 (2012).
De La Rochere, P. et al. Humanized mice for the study of immuno-oncology. Trends Immunol. 39, 748–763 (2018).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Kos, K. & de Visser, K. E. Neutrophils create a fertile soil for metastasis. Cancer Cell 39, 301–303 (2021).
Ronchetti, L. et al. Neutrophil extracellular traps in cancer: not only catching microbes. J. Exp. Clin. Cancer Res. 40, 231 (2021).
Arpinati, L. et al. NETosis in cancer: a critical analysis of the impact of cancer on neutrophil extracellular trap (NET) release in lung cancer patients vs. mice. Cancer Immunol. Immunother. 69, 199–213 (2020).
Tang, X. et al. Leukocyte-associated Ig-like receptor-1-deficient mice have an altered immune cell phenotype. J. Immunol. 188, 548–558 (2012).
Smith, C. W. et al. Mice lacking the inhibitory collagen receptor LAIR-1 exhibit a mild thrombocytosis and hyperactive platelets. Arterioscler. Thromb. Vasc. Biol. 37, 823–835 (2017).
Steevels, T. A. M. et al. Co-expression of collagen receptors LAIR-1 and GpVI on a subset of megakaryoblasts. Haematologica 95, 2005–2012 (2010).
Brondijk, T. H. et al. Crystal structure and collagen-binding site of immune inhibitory receptor LAIR-1: unexpected implications for collagen binding by platelet receptor GPVI. Blood 115, 1364–1373 (2010).
Helou, D. G. et al. LAIR-1 limits macrophage activation in acute inflammatory lung injury. Mucosal Immunol. 16, 788–800 (2023).
Gu, Y. et al. Expression and clinical significance of inhibitory receptor leukocyte-associated immunoglobulin-like receptor-1 on peripheral blood T cells of chronic hepatitis B patients: a cross-sectional study. Medicine 100, e26667 (2021).
Dorando, H. K. et al. LAIR1 prevents excess inflammatory tissue damage in S. aureus skin infection and cutaneous T-cell lymphoma. Preprint at bioRxiv https://doi.org/10.1101/2024.06.13.598864 (2024).
Zhang, Y. et al. The role of LAIR-1 (CD305) in T cells and monocytes/macrophages in patients with rheumatoid arthritis. Cell Immunol. 287, 46–52 (2014).
Colombo, B. M. et al. Defective expression and function of the leukocyte associated Ig-like receptor 1 in B lymphocytes from systemic lupus erythematosus patients. PLoS One 7, e31903 (2012).
Ellenbroek, G. et al. Leukocyte-associated immunoglobulin-like receptor-1 is regulated in human myocardial infarction but its absence does not affect infarct size in mice. Sci. Rep. 7, 18039 (2017).
Mori, S., Nagae, M. & Yamasaki, S. Crystal structure of the complex of CLEC12A and an antibody that interferes with binding of diverse ligands. Int. Immunol. 36, 279–290 (2024).
Chiang, E. Y. & Mellman, I. TIGIT–CD226–PVR axis: advancing immune checkpoint blockade for cancer immunotherapy. J. Immunother. Cancer 10, e004711 (2022).
Gonzalez-Gil, A., Li, T. A., Kim, J. & Schnaar, R. L. Human sialoglycan ligands for immune inhibitory Siglecs. Mol. Asp. Med. 90, 101110 (2023).
Jiang, X. et al. Oxidized galectin-1 in SLE fails to bind the inhibitory receptor VSTM1 and increases reactive oxygen species levels in neutrophils. Cell Mol. Immunol. 20, 1339–1351 (2023).
Poggi, A. et al. p40, a novel surface molecule involved in the regulation of the non-major histocompatibility complex-restricted cytolytic activity in humans. Eur. J. Immunol. 25, 369–376 (1995).
Ouyang, W. M. et al. 9.1C3 is identical to LAIR-1, which is expressed on hematopoietic progenitors. Biochem. Biophys. Res. Commun. 310, 1236–1240 (2003).
Poggi, A. et al. p40 molecule regulates NK cell activation mediated by NK receptors for HLA class I antigens and TCR-mediated triggering of T lymphocytes. Int. Immunol. 9, 1271–1279 (1997).
Jansen, C. A. et al. Regulated expression of the inhibitory receptor LAIR-1 on human peripheral T cells during T cell activation and differentiation. Eur. J. Immunol. 37, 914–924 (2007).
Saverino, D. et al. Surface density expression of the leukocyte-associated Ig-like receptor-1 is directly related to inhibition of human T-cell functions. Hum. Immunol. 63, 534–546 (2002).
Moir, S. et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205, 1797–1805 (2008).
Aiello, F. B. et al. IL-7-induced phosphorylation of the adaptor Crk-like and other targets. Cell Signal. 47, 131–141 (2018).
Poggi, A., Costa, P., Tomasello, E. & Moretta, L. IL-12-induced up-regulation of NKRP1A expression in human NK cells and consequent NKRP1A-mediated down-regulation of NK cell activation. Eur. J. Immunol. 28, 1611–1616 (1998).
Lee, M. Y. et al. Tissue-specific transcriptional profiling of plasmacytoid dendritic cells reveals a hyperactivated state in chronic SIV infection. PLoS Pathog. 17, e1009674 (2021).
Jin, J. et al. LAIR-1 activation inhibits inflammatory macrophage phenotype in vitro. Cell Immunol. 331, 78–84 (2018).
Rowley, A. T. et al. Effects of surface-bound collagen-mimetic peptides on macrophage uptake and immunomodulation. Front. Bioeng. Biotechnol. 8, 747 (2020).
Zhang, Y. et al. Expression of leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) on osteoclasts and its potential role in rheumatoid arthritis. Clinics 68, 475–481 (2013).
Boraschi-Diaz, I. et al. Collagen type I degradation fragments act through the collagen receptor LAIR-1 to provide a negative feedback for osteoclast formation. Bone 117, 23–30 (2018).
Besteman, S. B. et al. Signal inhibitory receptor on leukocytes (SIRL)-1 and leukocyte-associated immunoglobulin-like receptor (LAIR)-1 regulate neutrophil function in infants. Clin. Immunol. 211, 108324 (2020).
Acknowledgements
The authors thank all members of the Inhibitory Receptor Lab for their critical review and discussion of the manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The research laboratory at University Medical Center Utrecht has received research funding from NextCure, NGM Biopharmaceuticals, Boehringer Ingelheim and argenx. L.M. has received consultancy fees from Eli Lilly, Third Rock Ventures and Abbvie, all paid to the institute. M.I.P.R., L.M. and M.v.d.V. have not received personal fees or other personal benefits.
Peer review
Peer review information
Nature Reviews Immunology thanks Lihua Chen, Jacqueline Payton and Cheng Cheng Zhang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- C2-type immunoglobulin-like domain
-
A protein domain that shows evolutionary similarity, in both linear sequence and folded structure, to the immunoglobulin constant region type 2 (C2) domains. This structural motif is found in various proteins involved in cell adhesion, immune responses and signalling. As part of the immunoglobulin superfamily (IgSF), this domain has a characteristic β-sandwich fold, typically comprising two β-sheets that form a stable, compact structure.
- Carboxy-terminal Src-family kinase
-
(CSK). A non-receptor tyrosine kinase critical for regulating Src-family kinases (SFKs), a group of proteins that have key roles in cellular processes such as growth, differentiation and survival. By phosphorylating a conserved tyrosine residue in the C-terminal tail of SFKs, CSK inactivates these kinases. This inhibitory mechanism is vital for maintaining cellular homeostasis, as unchecked SFK activity is linked to cancer, immune disorders and other diseases. CSK is broadly expressed in mammalian cells and functions as a negative regulator, ensuring controlled activation of pathways related to cell motility, proliferation and adhesion.
- Damage-associated molecular patterns
-
(DAMPs). Endogenous molecules released by stressed, damaged or dying cells that alert the immune system to non-infectious tissue injury. Unlike pathogen-associated molecular patterns (PAMPs), which originate from microbial sources, DAMPs are host-derived signals. They are recognized by pattern recognition receptors (PRRs) on immune cells, such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs), leading to an inflammatory response aimed at containing tissue damage and promoting repair. Common examples of DAMPs include high-mobility group box 1 (HMGB1), ATP, heat shock proteins (HSPs) and extracellular DNA. DAMPs play an essential role in conditions such as trauma, autoimmune diseases and cancer by modulating inflammation and immune responses.
- Extracellular matrix
-
(ECM). The structural network of secreted proteins and glycosaminoglycans that provides structure to tissue.
- Immune-related adverse events
-
Toxic side effects that occur when therapies that block immune inhibitory receptors, such as anti-cytotoxic T lymphocyte antigen 4 (anti-CTLA4) and anti-PD1, are used to activate an anticancer immune response. Overactivation of the immune system with these therapies can cause harmful autoimmune attacks in other organs and tissues.
- Immunoreceptor tyrosine-based activation motif
-
(ITAM). A short peptide motif containing tyrosine residues that is found in the cytoplasmic tails of several activating receptors and in adaptors such as DAP12. The consensus sequence is (Asp/Glu)-X-X-Tyr-X-X-(Leu/Ile)-X6–8-Tyr-X-X-(Leu/Ile), with X denoting any amino acid. Tyrosine phosphorylation of this motif triggers a cascade of intracellular events that usually results in cellular activation.
- Immunoreceptor tyrosine-based inhibitory motifs
-
(ITIMs). Short peptide motifs containing tyrosine residues that are found in the cytoplasmic regions of many inhibitory receptors. The consensus sequence is (Ile/Val/Leu/Ser)-X-Tyr-X-X-(Leu/Val), with X denoting any amino acid. Tyrosine phosphorylation produces a binding site for cytoplasmic phosphatases and other signalling molecules, resulting in the dephosphorylation of activation complexes and inhibition of signalling cascades.
- Pathogen-associated molecular patterns
-
(PAMPs). Molecular components of pathogens that are not normally found in mammals and are often common to whole classes of microorganisms. PAMPs are sensed by host pattern recognition receptors (PRRs). Examples include β-glucan, which binds dectin-1, and various microbial products, such as bacterial lipopolysaccharides, hypomethylated DNA, flagellin and double-stranded RNA, which bind Toll-like receptors (TLRs).
- Pattern recognition receptor
-
(PRR). A receptor (such as Toll-like receptor (TLR)) that can sense pathogen-associated molecular pattern (PAMPs) or damage-associated molecular patterns (DAMPs) and initiate signalling cascades that lead to immune responses.
- Pre-metastatic niche
-
A microenvironment at a distant site that supports the survival and growth of disseminated tumour cells, preparing the site for their establishment even before their arrival.
- SHP1 and SHP2
-
SH2 domain-containing phosphatases that bind phosphorylated immunoreceptor tyrosine-based inhibitory motifs (ITIMs). The recruitment of SRC-homology region 2 domain-containing phosphatase 1 (SHP1) and SHP2 by ITIM-bearing receptors represents a general mechanism for modulation of immune cell signalling.
- Sialic acid-binding immunoglobulin-like lectins
-
(SIGLECs). A family of cell surface receptors that primarily recognize sialic acid-containing glycans, which are commonly found on the surface of cells. These receptors are involved in regulating immune cell signalling and interactions, particularly in controlling immune responses and maintaining self-tolerance.
- Single-nucleotide polymorphisms
-
(SNPs). Variations in a single nucleotide (the building blocks of DNA) in the genome that occur at a specific position in the DNA sequence among individuals of a species. These variations are common in the human population and can have implications for genetic diversity, disease susceptibility and drug responses.
- Tumour microenvironment
-
(TME). A complex and dynamic ecosystem surrounding a tumour, comprising a diverse network of cells, molecules and structures that interact with the tumour to shape its growth, progression and response to therapies.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pascoal Ramos, M.I., van der Vlist, M. & Meyaard, L. Inhibitory pattern recognition receptors: lessons from LAIR1. Nat Rev Immunol 25, 711–724 (2025). https://doi.org/10.1038/s41577-025-01181-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-025-01181-2