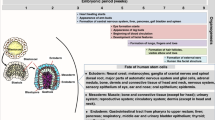
Abstract
During pregnancy, the maternal immune system must navigate a balance between fetal tolerance and a response to acquired microbial infections. Cells at the maternal–fetal interface coordinate this response throughout gestational stages in a dynamic manner, integrating endocrine, developmental, inflammatory and metabolic cues. Although many maternal and fetal cell types activate innate immune signalling pathways in response to infections, excessive inflammation can disrupt tolerance, affect placental function and impair fetal development, leading to congenital disease and other pregnancy complications. In this Review, we discuss the mechanisms of pathogen recognition in the maternal and fetal compartments of the placenta and the consequences of these responses to pregnancy outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Devaraju, M. et al. Beyond TORCH: a narrative review of the impact of antenatal and perinatal infections on the risk of disability. Neurosci. Biobehav. Rev. 153, 105390 (2023).
Ander, S. E., Diamond, M. S. & Coyne, C. B. Immune responses at the maternal–fetal interface. Sci. Immunol. 4, eaat6114 (2019).
Espino, A., El Costa, H., Tabiasco, J., Al-Daccak, R. & Jabrane-Ferrat, N. Innate immune response to viral infections at the maternal–fetal interface in human pregnancy. Front. Med. 8, 674645 (2021).
Hoo, R., Nakimuli, A. & Vento-Tormo, R. Innate immune mechanisms to protect against infection at the human decidual–placental interface. Front. Immunol. 11, 2070 (2020).
Semmes, E. C. & Coyne, C. B. Innate immune defenses at the maternal–fetal interface. Curr. Opin. Immunol. 74, 60–67 (2022).
Arutyunyan, A. et al. Spatial multiomics map of trophoblast development in early pregnancy. Nature 616, 143–151 (2023).
Greenbaum, S. et al. A spatially resolved timeline of the human maternal–fetal interface. Nature 619, 595–605 (2023).
Moore, A. R. et al. Gestationally dependent immune organization at the maternal–fetal interface. Cell Rep. 41, 111651 (2022). This paper describes the immuno-phenotyping of circulating, endovascular and tissue-resident cells at the maternal–fetal interface throughout gestation, and distinguishes maternal and fetal contributions.
Gauster, M., Moser, G., Wernitznig, S., Kupper, N. & Huppertz, B. Early human trophoblast development: from morphology to function. Cell Mol. Life Sci. 79, 345 (2022).
Arranz-Solis, D., Mukhopadhyay, D. & Saeij, J. J. P. Toxoplasma effectors that affect pregnancy outcome. Trends Parasitol. 37, 283–295 (2021).
Schatten, H. & Constantinescu, G. M. Comparative Reproductive Biology 1st edn (Blackwell, 2007).
Wooding, F. B. P. & Burton, G. Comparative Placentation: Structures, Functions and Evolution (Springer, 2008).
Kingdom, J., Huppertz, B., Seaward, G. & Kaufmann, P. Development of the placental villous tree and its consequences for fetal growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 92, 35–43 (2000).
Reyes, L. & Golos, T. G. Hofbauer cells: their role in healthy and complicated pregnancy. Front. Immunol. 9, 2628 (2018).
Loegl, J. et al. Hofbauer cells of M2a, M2b and M2c polarization may regulate feto-placental angiogenesis. Reproduction 152, 447–455 (2016).
Thomas, J. R., Naidu, P., Appios, A. & McGovern, N. The ontogeny and function of placental macrophages. Front. Immunol. 12, 771054 (2021).
Guttman, J. A. & Finlay, B. B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 1788, 832–841 (2009).
Ander, S. E. et al. Human placental syncytiotrophoblasts restrict Toxoplasma gondii attachment and replication and respond to infection by producing immunomodulatory chemokines. mBio 9, e01678-17 (2018).
Robbins, J. R., Zeldovich, V. B., Poukchanski, A., Boothroyd, J. C. & Bakardjiev, A. I. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect. Immun. 80, 418–428 (2012).
Robbins, J. R., Skrzypczynska, K. M., Zeldovich, V. B., Kapidzic, M. & Bakardjiev, A. I. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 6, e1000732 (2010).
Zeldovich, V. B. et al. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog. 9, e1003821 (2013).
Bayer, A. et al. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 19, 705–712 (2016).
Corry, J., Arora, N., Good, C. A., Sadovsky, Y. & Coyne, C. B. Organotypic models of type III interferon-mediated protection from Zika virus infections at the maternal–fetal interface. Proc. Natl Acad. Sci. USA 114, 9433–9438 (2017).
Wickramage, I. et al. SINE RNA of the imprinted miRNA clusters mediates constitutive type III interferon expression and antiviral protection in hemochorial placentas. Cell Host Microbe 31, 1185–1199.e10 (2023). This paper reveals that dsRNA produced by imprinted placental microRNA clusters in primates and rodents triggers constitutive type III interferon production, conferring innate antiviral protection in these haemochorial placentas.
Yang, L. et al. Innate immune signaling in trophoblast and decidua organoids defines differential antiviral defenses at the maternal–fetal interface. eLife 11, e79794 (2022). This study defines the innate immunological properties of trophoblast and decidua organoids derived from term placental tissue, revealing distinct responses to viral infection and differential susceptibility to CMV.
Burton, G. J., Watson, A. L., Hempstock, J., Skepper, J. N. & Jauniaux, E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J. Clin. Endocrinol. Metab. 87, 2954–2959 (2002).
Pollheimer, J., Vondra, S., Baltayeva, J., Beristain, A. G. & Knofler, M. Regulation of placental extravillous trophoblasts by the maternal uterine environment. Front. Immunol. 9, 2597 (2018).
Apps, R., Gardner, L., Sharkey, A. M., Holmes, N. & Moffett, A. A homodimeric complex of HLA-G on normal trophoblast cells modulates antigen-presenting cells via LILRB1. Eur. J. Immunol. 37, 1924–1937 (2007).
Chen, L. J., Han, Z. Q., Zhou, H., Zou, L. & Zou, P. Inhibition of HLA-G expression via RNAi abolishes resistance of extravillous trophoblast cell line TEV-1 to NK lysis. Placenta 31, 519–527 (2010).
Chumbley, G., King, A., Robertson, K., Holmes, N. & Loke, Y. W. Resistance of HLA-G and HLA-A2 transfectants to lysis by decidual NK cells. Cell Immunol. 155, 312–322 (1994).
Yang, Y. et al. Advances in the study of HLA class Ib in maternal–fetal immune tolerance. Front. Immunol. 13, 976289 (2022).
Mori, M., Bogdan, A., Balassa, T., Csabai, T. & Szekeres-Bartho, J. The decidua—the maternal bed embracing the embryo-maintains the pregnancy. Semin. Immunopathol. 38, 635–649 (2016).
Vento-Tormo, R. et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 563, 347–353 (2018). This publication profiles the first-trimester human maternal–fetal interface using single-cell RNA sequencing, revealing complex interactions between maternal and fetal cell populations, including identifying the distinct immunological properties of dNK cell subsets.
Carlino, C. et al. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood 111, 3108–3115 (2008).
Huang, Y., Zhu, X. Y., Du, M. R. & Li, D. J. Human trophoblasts recruited T lymphocytes and monocytes into decidua by secretion of chemokine CXCL16 and interaction with CXCR6 in the first-trimester pregnancy. J. Immunol. 180, 2367–2375 (2008).
Manaster, I. & Mandelboim, O. The unique properties of uterine NK cells. Am. J. Reprod. Immunol. 63, 434–444 (2010).
Thomas, J. R. et al. Phenotypic and functional characterization of first-trimester human placental macrophages, Hofbauer cells. J. Exp. Med. 218, e20200891 (2020). This study provides a detailed characterization of first-trimester HBCs, demonstrating their transcriptional similarity to yolk sac macrophages, capacity to secrete factors involved in placental angiogenesis and remodelling, and microbicidal defence mechanisms.
Rowe, J. H., Ertelt, J. M., Xin, L. & Way, S. S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490, 102–106 (2012).
Aluvihare, V. R., Kallikourdis, M. & Betz, A. G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271 (2004).
Jasper, M. J., Tremellen, K. P. & Robertson, S. A. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol. Hum. Reprod. 12, 301–308 (2006).
Sket, T., Ramuta, T. Z., Starcic Erjavec, M. & Kreft, M. E. The role of innate immune system in the human amniotic membrane and human amniotic fluid in protection against intra-amniotic infections and inflammation. Front. Immunol. 12, 735324 (2021).
Marsh, B., Zhou, Y., Kapidzic, M., Fisher, S. & Blelloch, R. Regionally distinct trophoblast regulate barrier function and invasion in the human placenta. eLife 11, e78829 (2022).
Carter, A. M. & Pijnenborg, R. Evolution of invasive placentation with special reference to non-human primates. Best. Pract. Res. Clin. Obstet. Gynaecol. 25, 249–257 (2011).
Yong, H. E. J. et al. Significance of the placental barrier in antenatal viral infections. Biochim. Biophys. Acta - Mol. Basis Dis. 1867, 166244 (2021).
Kumar, M., Saadaoui, M. & Al Khodor, S. Infections and pregnancy: effects on maternal and child health. Front. Cell. Infect. Microbiol. 12, 873253 (2022).
Yates, E. F. & Mulkey, S. B. Viral infections in pregnancy and impact on offspring neurodevelopment: mechanisms and lessons learned. Pediatr. Res. 96, 64–72 (2024).
Enders, G., Daiminger, A., Bader, U., Exler, S. & Enders, M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J. Clin. Virol. 52, 244–246 (2011).
Noronha, L. D. et al. Zika virus infection at different pregnancy stages: anatomopathological findings, target cells and viral persistence in placental tissues. Front. Microbiol. 9, 2266 (2018).
Creisher Patrick, S. & Klein Sabra, L. Pathogenesis of viral infections during pregnancy. Clin. Microbiol. Rev. 37, e0007323 (2024).
Megli, C. J. & Coyne, C. B. Infections at the maternal–fetal interface: an overview of pathogenesis and defence. Nat. Rev. Microbiol. 20, 67–82 (2022).
Cruz-Holguín, V. J. et al. Collateral damage in the placenta during viral infection in pregnancy: a possible mechanism for vertical transmission and an adverse pregnancy outcome. Diseases 12, 59 (2024).
Neu, N., Duchon, J. & Zachariah, P. TORCH infections. Clin. Perinatol. 42, 77–103 (2015).
Pereira, L., Maidji, E., McDonagh, S., Genbacev, O. & Fisher, S. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J. Virol. 77, 13301–13314 (2003).
Aronoff, D. M., Correa, H., Rogers, L. M., Arav-Boger, R. & Alcendor, D. J. Placental pericytes and cytomegalovirus infectivity: implications for HCMV placental pathology and congenital disease. Am. J. Reprod. Immunol. 78, e12728 (2017).
Njue, A. et al. The role of congenital cytomegalovirus infection in adverse birth outcomes: a review of the potential mechanisms. Viruses 13, 20 (2021).
Uenaka, M. et al. Histopathological analysis of placentas with congenital cytomegalovirus infection. Placenta 75, 62–67 (2019).
Miner, J. J. & Diamond, M. S. Zika virus pathogenesis and tissue tropism. Cell Host Microbe 21, 134–142 (2017).
Sheridan, M. A. et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc. Natl Acad. Sci. USA 114, E1587–E1596 (2017).
Tabata, T. et al. Zika virus replicates in proliferating cells in explants from first-trimester human placentas, potential sites for dissemination of infection. J. Infect. Dis. 217, 1202–1213 (2017).
Weisblum, Y. et al. Zika virus infects early- and midgestation human maternal decidual tissues, inducing distinct innate tissue responses in the maternal–fetal interface. J. Virol. 91, 01905-16 (2017).
Venceslau, E. M., Guida, J. P., Amaral, E., Modena, J. L. P. & Costa, M. L. Characterization of placental infection by Zika virus in humans: a review of the literature. Rev. Bras. Ginecol. Obstet. 42, 577–585 (2020).
Yockey, L. J. et al. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell 166, 1247–1256.e4 (2016).
Sankaran, D., Partridge, E. & Lakshminrusimha, S. Congenital syphilis—an illustrative review. Children 10, 1310 (2023).
Vazquez-Boland, J. A., Krypotou, E. & Scortti, M. Listeria placental infection. mBio 8, e13186 (2017).
Parkash, V. et al. Immunohistochemical detection of Listeria antigens in the placenta in perinatal listeriosis. Int. J. Gynecol. Pathol. 17, 343–350 (1998).
Sheffield, J. S. et al. Placental histopathology of congenital syphilis. Obstet. Gynecol. 100, 126–133 (2002).
Eallonardo, S. J. & Freitag, N. E. Crossing the barrier: a comparative study of Listeria monocytogenes and Treponema pallidum in placental invasion. Cells 13, 88 (2023).
Bakardjiev, A. I., Stacy, B. A. & Portnoy, D. A. Growth of Listeria monocytogenes in the guinea pig placenta and role of cell-to-cell spread in fetal infection. J. Infect. Dis. 191, 1889–1897 (2005).
Critchfield, A. S. et al. Cervical mucus properties stratify risk for preterm birth. PLoS One 8, e69528 (2013).
Smith-Dupont, K. B. et al. Probing the potential of mucus permeability to signify preterm birth risk. Sci. Rep. 7, 10302 (2017).
Hoang, T. et al. The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis. PLoS Pathog. 16, e1008236 (2020).
Brokaw, A., Furuta, A., Dacanay, M., Rajagopal, L. & Adams Waldorf, K. M. Bacterial and host determinants of group B streptococcal vaginal colonization and ascending infection in pregnancy. Front. Cell Infect. Microbiol. 11, 720789 (2021).
Ruma, M. et al. Maternal periodontal disease, systemic inflammation, and risk for preeclampsia. Am. J. Obstet. Gynecol. 198, 389.e1–e5 (2008).
Bollani, L. et al. Congenital toxoplasmosis: the state of the art. Front. Pediatr. 10, 894573 (2022).
Kemmerling, U., Osuna, A., Schijman, A. G. & Truyens, C. Congenital transmission of Trypanosoma cruzi: a review about the interactions between the parasite, the placenta, the maternal and the fetal/neonatal immune responses. Front. Microbiol. 10, 1854 (2019).
Torrico, F. et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am. J. Trop. Med. Hyg. 70, 201–209 (2004).
Altemani, A. M., Bittencourt, A. L. & Lana, A. M. Immunohistochemical characterization of the inflammatory infiltrate in placental Chagas’ disease: a qualitative and quantitative analysis. Am. J. Trop. Med. Hyg. 62, 319–324 (2000).
Hasby Saad, M., El-Anwar, N., Lotfy, S., Fouda, M. & Hasby, E. Human placental PPAR-γ and SOX-2 expression in serologically proved toxoplasmosis. Parasite Immunol. 40, e12529 (2018).
Mjihdi, A. et al. Acute Trypanosoma cruzi infection in mouse induces infertility or placental parasite invasion and ischemic necrosis associated with massive fetal loss. Am. J. Pathol. 161, 673–680 (2002).
Duaso, J. et al. Trypanosoma cruzi induces tissue disorganization and destruction of chorionic villi in an ex vivo infection model of human placenta. Placenta 31, 705–711 (2010).
Duaso, J. et al. Trypanosoma cruzi induces apoptosis in ex vivo infected human chorionic villi. Placenta 32, 356–361 (2011).
Bauserman, M. et al. An overview of malaria in pregnancy. Semin. Perinatol. 43, 282–290 (2019).
Koga, K. & Mor, G. Review article: Toll-like receptors at the maternal–fetal interface in normal pregnancy and pregnancy disorders. Am. J. Reprod. Immunol. 63, 587–600 (2010).
Motomura, K., Hara, M., Ito, I., Morita, H. & Matsumoto, K. Roles of human trophoblasts’ pattern recognition receptors in host defense and pregnancy complications. J. Reprod. Immunol. 156, 103811 (2023).
Mysorekar, I. U. & Diamond, M. S. Modeling Zika virus infection in pregnancy. N. Engl. J. Med. 375, 481–484 (2016).
Kliman, H. J., Nestler, J. E., Sermasi, E., Sanger, J. M. & Strauss, J. F. 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118, 1567–1582 (1986).
Okae, H. et al. Derivation of human trophoblast stem cells. Cell Stem Cell 22, 50–63.e6 (2018). This paper describes the derivation of human TSCs from early placental CTBs and blastocysts, yielding a self-renewing model that can be stimulated to differentiate into the three major trophoblast lineages.
Sheridan, M. A. et al. Characterization of primary models of human trophoblast. Development 148, dev199749 (2021).
Hori, T. et al. Trophoblast stem cell-based organoid models of the human placental barrier. Nat. Commun. 15, 962 (2024).
Turco, M. Y. et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 19, 568–577 (2017).
Turco, M. Y. et al. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 564, 263–267 (2018). This study demonstrates that trophoblast organoids isolated from first-trimester placental tissue recapitulate the transcriptomic and methylation profiles of early-gestation trophoblasts and spontaneously differentiate into CTBs, EVTs and STBs.
Rollman, T. B. et al. Human trophoblast stem cells restrict human cytomegalovirus replication. J. Virol. 98, e0193523 (2024).
Wu, H. et al. Zika virus targets human trophoblast stem cells and prevents syncytialization in placental trophoblast organoids. Nat. Commun. 14, 5541 (2023).
da Silva, R. J. et al. The trophoblast surface becomes refractory to adhesion by congenitally transmitted Toxoplasma gondii and Listeria monocytogenes during cytotrophoblast to syncytiotrophoblast development. mSphere 9, e00748-23 (2024).
Karvas, R. M. et al. Stem-cell-derived trophoblast organoids model human placental development and susceptibility to emerging pathogens. Cell Stem Cell 29, 810–825.e8 (2022).
Morrison, T. E. & Diamond, M. S. Animal models of Zika virus infection, pathogenesis, and immunity. J. Virol. 91, e00009-17 (2017).
Narasimhan, H., Chudnovets, A., Burd, I., Pekosz, A. & Klein, S. L. Animal models of congenital Zika syndrome provide mechanistic insight into viral pathogenesis during pregnancy. PLoS Negl. Trop. Dis. 14, e0008707 (2020).
Koenig, M. R. et al. Vertical transmission of African-lineage Zika virus through the fetal membranes in a rhesus macaque (Macaca mulatta) model. PLoS Pathog. 19, e1011274 (2023).
Itell, H. L., Kaur, A., Deere, J. D., Barry, P. A. & Permar, S. R. Rhesus monkeys for a nonhuman primate model of cytomegalovirus infections. Curr. Opin. Virol. 25, 126–133 (2017).
Moström, M. J. et al. Protective effect of pre-existing natural immunity in a nonhuman primate reinfection model of congenital cytomegalovirus infection. PLoS Pathog. 19, e1011646 (2023).
Bialas, K. M. et al. Maternal CD4+ T cells protect against severe congenital cytomegalovirus disease in a novel nonhuman primate model of placental cytomegalovirus transmission. Proc. Natl Acad. Sci. USA 112, 13645–13650 (2015).
Schleiss, M. R. & McVoy, M. A. Guinea pig cytomegalovirus: a model for the prevention and treatment of maternal–fetal cytomegalovirus transmission. Future Virol. 5, 207–217 (2010).
Griffith, B. P. et al. The placenta as a site of cytomegalovirus infection in guinea pigs. J. Virol. 55, 402–409 (1985).
Mess, A. The guinea pig placenta: model of placental growth dynamics. Placenta 28, 812–815 (2007).
Berkebile, Z. W. et al. The placental response to guinea pig cytomegalovirus depends upon the timing of maternal infection. Front. Immunol. 12, 686415 (2021).
Rollman, T. B. et al. CD4+ but not CD8+ T cells are required for protection against severe guinea pig cytomegalovirus infections. PLOS Pathog. 20, e1012515 (2024).
Koga, K. et al. Original article: activation of TLR3 in the trophoblast is associated with preterm delivery. Am. J. Reprod. Immunol. 61, 196–212 (2009). This study shows that activation of TLR3 in trophoblasts is associated with an increased risk of preterm delivery in mice, potentially through induction of inflammatory responses at the maternal–fetal interface.
Robertson, S. A. et al. Targeting Toll-like receptor-4 to tackle preterm birth and fetal inflammatory injury. Clin. Transl. Immunol. 9, e1121 (2020).
Arce, R. M., Caron, K. M., Barros, S. P. & Offenbacher, S. Toll-like receptor 4 mediates intrauterine growth restriction after systemic Campylobacter rectus infection in mice. Mol. Oral. Microbiol. 27, 373–381 (2012).
Liu, H., Redline, R. W. & Han, Y. W. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response1. J. Immunol. 179, 2501–2508 (2007).
Barboza, R. et al. TLR4-mediated placental pathology and pregnancy outcome in experimental malaria. Sci. Rep. 7, 8623 (2017).
Rodrigues-Duarte, L., Pandya, Y., Neres, R. & Penha-Gonçalves, C. Fetal and maternal innate immunity receptors have opposing effects on the severity of experimental malaria in pregnancy: beneficial roles for fetus-derived Toll-like receptor 4 and type I interferon receptor 1. Infect. Immun. 86, e00708-17 (2018).
Ikeda, R., Ushio, N., Abdou, A. M., Furuoka, H. & Nishikawa, Y. Toll-like receptor 2 is involved in abnormal pregnancy in mice infected with Toxoplasma gondii during late pregnancy. Front. Microbiol. 12, 741104 (2021).
Gorman, M. J. et al. An immunocompetent mouse model of Zika virus infection. Cell Host Microbe 23, 672–685.e6 (2018).
Grant, A. et al. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe 19, 882–890 (2016).
Szaba, F. M. et al. Zika virus infection in immunocompetent pregnant mice causes fetal damage and placental pathology in the absence of fetal infection. PLoS Pathog. 14, e1006994 (2018).
Cugola, F. R. et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267–271 (2016).
Lazear, H. M. et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe 19, 720–730 (2016).
Miner, J. J. et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165, 1081–1091 (2016).
Alippe, Y. et al. Fetal MAVS and type I IFN signaling pathways control ZIKV infection in the placenta and maternal decidua. J. Exp. Med. 221, e20240694 (2024).
Casazza, R. L., Philip, D. T. & Lazear, H. M. Interferon λ signals in maternal tissues to exert protective and pathogenic effects in a gestational stage-dependent manner. mBio 13, e0385721 (2022). This paper demonstrates that in mice, IFNλ signalling in maternal tissues can exert both protective and pathogenic effects on congenital ZIKV infection depending on the gestational stage.
Jagger, B. W. et al. Gestational stage and IFN-λ signaling regulate ZIKV infection in utero. Cell Host Microbe 22, 366–376.e3 (2017).
Motomura, K. et al. Fetal and maternal NLRP3 signaling is required for preterm labor and birth. JCI Insight 7, e158238 (2022).
Racicot, K. et al. Cutting edge: fetal/placental type I IFN can affect maternal survival and fetal viral load during viral infection. J. Immunol. 198, 3029–3032 (2017).
Medzhitov, R. The spectrum of inflammatory responses. Science 374, 1070–1075 (2021).
Thaxton, J. E., Romero, R. & Sharma, S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes1. J. Immunol. 183, 1144–1154 (2009).
Riley, J. K., Heeley, J. M., Wyman, A. H., Schlichting, E. L. & Moley, K. H. TRAIL and KILLER are expressed and induce apoptosis in the murine preimplantation embryo1. Biol. Reprod. 71, 871–877 (2004).
Yockey, L. J. et al. Type I interferons instigate fetal demise after Zika virus infection. Sci. Immunol. 3, eaao1680 (2018).
Aldo, P. B., Mulla, M. J., Romero, R., Mor, G. & Abrahams, V. M. Viral ssRNA induces first trimester trophoblast apoptosis through an inflammatory mechanism. Am. J. Reprod. Immunol. 64, 27–37 (2010).
Choi, G. B. et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351, 933–939 (2016).
Smith, S. E., Li, J., Garbett, K., Mirnics, K. & Patterson, P. H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 27, 10695–10702 (2007).
Chaturvedi, V. et al. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J. Clin. Invest. 125, 1713–1725 (2015). This publication shows that CXCR3 blockade can protect against fetal wastage and prevent the accumulation of maternal T cells with fetal specificity in the decidua following L. monocytogenes infection.
Ma, J. et al. Zika virus non-structural protein 4 A blocks the RLR–MAVS signaling. Front. Microbiol. 9, 1350 (2018).
Zhao, Z. et al. Zika virus causes placental pyroptosis and associated adverse fetal outcomes by activating GSDME. eLife 11, e73792 (2022).
You, Y. et al. Twist1–IRF9 interaction is necessary for IFN-stimulated gene anti-Zika viral infection. J. Immunol. 210, 1899–1912 (2023).
Coldbeck-Shackley, R. C. et al. Constitutive expression and distinct properties of IFN-ε protect the female reproductive tract from Zika virus infection. PLOS Pathog. 19, e1010843 (2023).
Abrahams, V. M. et al. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, poly(I:C). Hum. Reprod. 21, 2432–2439 (2006).
Abrahams, V. M. et al. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J. Immunol. 175, 8096–8104 (2005).
Gargano, L. M., Moser, J. M. & Speck, S. H. Role for MyD88 signaling in murine gammaherpesvirus 68 latency. J. Virol. 82, 3853–3863 (2008).
Cardenas, I. et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J. Immunol. 185, 1248–1257 (2010).
Barboza, R. et al. MyD88 signaling is directly involved in the development of murine placental malaria. Infect. Immun. 82, 830–838 (2014).
Senegas, A. et al. Toxoplasma gondii-induced foetal resorption in mice involves interferon-γ-induced apoptosis and spiral artery dilation at the maternofoetal interface. Int. J. Parasitol. 39, 481–487 (2009).
Duriez, M. et al. Human decidual macrophages and NK cells differentially express Toll-like receptors and display distinct cytokine profiles upon TLR stimulation. Front. Microbiol. https://doi.org/10.3389/fmicb.2014.00316 (2014).
Anders, A. P., Gaddy, J. A., Doster, R. S. & Aronoff, D. M. Current concepts in maternal–fetal immunology: recognition and response to microbial pathogens by decidual stromal cells. Am. J. Reprod. Immunol. 77, e12623 (2017).
Matias, M. L. et al. Endogenous and uric acid-induced activation of NLRP3 inflammasome in pregnant women with preeclampsia. PLoS One 10, e0129095 (2015).
Gomez-Lopez, N. et al. A role for the inflammasome in spontaneous labor at term with acute histologic chorioamnionitis. Reprod. Sci. 24, 934–953 (2017).
Megli, C., Morosky, S., Rajasundaram, D. & Coyne, C. B. Inflammasome signaling in human placental trophoblasts regulates immune defense against Listeria monocytogenes infection. J. Exp. Med. 218, e20200649 (2020).
Murphy, S. P., Fast, L. D., Hanna, N. N. & Sharma, S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J. Immunol. 175, 4084–4090 (2005).
Cheng, S. B. & Sharma, S. Interleukin-10: a pleiotropic regulator in pregnancy. Am. J. Reprod. Immunol. 73, 487–500 (2015).
Svensson-Arvelund, J. et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J. Immunol. 194, 1534–1544 (2015).
Robertson, S. A., Skinner, R. J. & Care, A. S. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J. Immunol. 177, 4888–4896 (2006).
Robertson, S. A., Care, A. S. & Skinner, R. J. Interleukin 10 regulates inflammatory cytokine synthesis to protect against lipopolysaccharide-induced abortion and fetal growth restriction in mice. Biol. Reprod. 76, 738–748 (2007).
Crespo, Â. C. et al. Decidual NK cells transfer granulysin to selectively kill bacteria in trophoblasts. Cell 182, 1125–1139.e18 (2020). This study demonstrates that dNK cells in the placenta transfer granulysin to trophoblasts, selectively killing bacteria without harming the trophoblast cells themselves.
Buchrieser, J. et al. IFITM proteins inhibit placental syncytiotrophoblast formation and promote fetal demise. Science 365, 176–180 (2019).
Zani, A. et al. Interferon-induced transmembrane proteins inhibit cell fusion mediated by trophoblast syncytins. J. Biol. Chem. 294, 19844–19851 (2019).
Vota, D. et al. Zika virus infection of first trimester trophoblast cells affects cell migration, metabolism and immune homeostasis control. J. Cell. Physiol. 236, 4913–4925 (2021).
Yockey, L. J. & Iwasaki, A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 49, 397–412 (2018).
Moldenhauer, L. M., Hull, M. L., Foyle, K. L., McCormack, C. D. & Robertson, S. A. Immune–metabolic interactions and T cell tolerance in pregnancy. J. Immunol. 209, 1426–1436 (2022).
Marshall, E. E. & Geballe, A. P. Multifaceted evasion of the interferon response by cytomegalovirus. J. Interferon Cytokine Res. 29, 609–619 (2009).
Fatemi, S. H. et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol. Neurobiol. 22, 25–33 (2002).
Shi, L., Tu, N. & Patterson, P. H. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int. J. Dev. Neurosci. 23, 299–305 (2005).
Ashary, N. et al. Single-cell RNA-seq identifies cell subsets in human placenta that highly expresses factors driving pathogenesis of SARS-CoV-2. Front. Cell Dev. Biol. 8, 783 (2020).
Barrozo, E. R. et al. SARS-CoV-2 niches in human placenta revealed by spatial transcriptomics. Med 4, 612–634.e4 (2023).
Wang, Y. et al. Cell-type specific distribution and activation of type I IFN pathway molecules at the placental maternal–fetal interface in response to COVID-19 infection. Front. Endocrinol. 13, 951388 (2023).
Schwartz, D. A., Dashraath, P. & Baud, D. Oropouche virus (OROV) in pregnancy: an emerging cause of placental and fetal infection associated with stillbirth and microcephaly following vertical transmission. Viruses 16, 1435 (2024).
Castro, M. C. & Lima Neto, A. S. Unprecedented spread and genetic evolution of the Oropouche virus. Nat. Med. https://doi.org/10.1038/s41591-024-03336-5 (2024).
Gaytant, M. A., Steegers, E. A., Semmekrot, B. A., Merkus, H. M. & Galama, J. M. Congenital cytomegalovirus infection: review of the epidemiology and outcome. Obstet. Gynecol. Surv. 57, 245–256 (2002).
Mahant, S. et al. Neonatal herpes simplex virus infection among medicaid-enrolled children: 2009–2015. Pediatrics 143, e20183233 (2019).
Shi, T. L. et al. The risk of herpes simplex virus and human cytomegalovirus infection during pregnancy upon adverse pregnancy outcomes: a meta-analysis. J. Clin. Virol. 104, 48–55 (2018).
Felker, A. M., Nguyen, P. & Kaushic, C. Primary HSV-2 infection in early pregnancy results in transplacental viral transmission and dose-dependent adverse pregnancy outcomes in a novel mouse model. Viruses 13, 1929 (2021).
Lamont, R. F. et al. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG 118, 1155–1162 (2011).
Robertson, N. J. & McKeever, P. A. Fetal and placental pathology in two cases of maternal varicella infection. Pediatr. Pathol. 12, 545–550 (1992).
Winter, A. K. & Moss, W. J. Rubella. Lancet 399, 1336–1346 (2022).
Lazar, M. et al. Immunolocalization and distribution of rubella antigen in fatal congenital rubella syndrome. EBioMedicine 3, 86–92 (2016).
Mulkey, S. B. et al. Neurodevelopmental abnormalities in children with in utero zika virus exposure without congenital zika syndrome. JAMA Pediatr. 174, 269–276 (2020).
Brasil, P. et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 375, 2321–2334 (2016).
Wedi, C. O. et al. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV 3, e33–e48 (2016).
Amin, O., Powers, J., Bricker, K. M. & Chahroudi, A. Understanding viral and immune interplay during vertical transmission of HIV: implications for cure. Front. Immunol. 12, 757400 (2021).
Bruce-Brand, C., Wright, C. A. & Schubert, P. T. HIV, placental pathology, and birth outcomes—a brief overview. J. Infect. Dis. 224, S683–S690 (2021).
Di Martino, V. et al. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology 40, 1426–1433 (2004).
Vousden, N., Bunch, K. & Knight, M. Incidence, risk factors and impact of seasonal influenza in pregnancy: a national cohort study. PLoS One 16, e0244986 (2021).
He, J. et al. A systematic review and meta-analysis of influenza A virus infection during pregnancy associated with an increased risk for stillbirth and low birth weight. Kidney Blood Press. Res. 42, 232–243 (2017).
Badr, D. A. et al. Severe acute respiratory syndrome coronavirus 2 and pregnancy outcomes according to gestational age at time of infection. Emerg. Infect. Dis. 27, 2535–2543 (2021).
Wei, S. Q., Bilodeau-Bertrand, M., Liu, S. & Auger, N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 193, E540–E548 (2021).
Stenton, S. et al. SARS-COV2 placentitis and pregnancy outcome: a multicentre experience during the Alpha and early Delta waves of coronavirus pandemic in England. EClinicalMedicine 47, 101389 (2022).
Acknowledgements
The authors acknowledge US National Institutes of Health grant R01AI145828 for support.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
M.S.D. is a consultant or on a Scientific Advisory Board for Inbios, IntegerBio, Akagera Medicines, GlaxoSmithKline, Merck and Moderna. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Vir Biotechnology, Emergent BioSolutions, Bavarian Nordic and Moderna.
Peer review
Peer review information
Nature Reviews Immunology thanks I. Messaoudi, G. Mor and S. A. Robertson for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Decidualization
-
The functional and morphological transformation that occurs within the endometrium. Decidualization encompasses endocrine, biochemical and immunological changes to form the decidual lining that allows blastocyst implantation. It involves the recruitment of leukocytes and the differentiation of endometrial fibroblasts into terminally differentiated decidual stromal cells. Decidualization includes the repression of genes involved in inflammation and the induction of genes that promote cellular proliferation and angiogenesis, immune tolerance and tissue invasion.
- Epiblast
-
(Also known as the primitive ectoderm). One of the two cell layers derived from the inner cell mass of the blastocyst. During gastrulation, the epiblast differentiates into the three primary germ layers (ectoderm, mesoderm and endoderm). The other layer of the inner cell mass of the epiblast, the hypoblast, gives rise to the yolk sac, which in turn contributes to the generation of the chorion.
- Interferon-λ
-
(IFNλ; also referred as type III interferon or IL28/29). A multi-gene family of antiviral cytokines (IFNL1, IFNL2, IFNL3, IFNL4) that share similar responses with type I interferons (for example, IFNα/β). Both type I and type III interferons are produced in response to pathogen-associated molecular pattern (PAMP)-generated microbial infections and activation of pattern-recognition receptors (PRRs). Paracrine or autocrine engagement of the heterodimeric IFNλ receptor (IL28Rα/IL10Rβ) by IFNλ results in activation of JAK/STAT signalling pathways, nuclear translocation of STAT1 and STAT2, and transcriptional induction of hundreds of interferon-stimulated genes (ISGs). Because of cell type and tissue-limited restriction of the IL28Rα subunit, antiviral responses by IFNλ are elicited preferentially at barrier surfaces, including the skin, the respiratory and intestinal tracts, and the maternal–fetal interface. In contrast to type I interferons, IFNλ responses are less inflammatory, but in some cases often more sustained, suggesting complementary actions of these two set of cytokines in the response to viral infections at tissue barrier sites.
- ‘M2-like’ macrophages
-
‘M1’ and ‘M2’ are terms used to define macrophages activated in vitro as pro-inflammatory (when exposed to interferon-γ (IFNγ) or lipopolysaccharide (LPS)) or anti-inflammatory (when ‘alternatively’ activated with IL-4 or IL-10). In vivo macrophages are highly specialized, transcriptomically dynamic and heterogeneous, and the M1 or M2 classification is too simplistic to explain their functional status. However, these terms are still often used to indicate whether the macrophages in question are more pro-inflammatory or anti-inflammatory, respectively.
- Oropouche virus
-
Orthobunyavirus oropoucheense is an arthropod-transmitted tripartite, negative-sense RNA virus in the Peribunyaviridae family. Infection in humans causes Oropouche fever in Latin America and the Caribbean, a mild and self-limited febrile illness that wanes after a week. However, some individuals progress to haemorrhagic manifestations or neurological sequelae from encephalitis, which can cause considerable morbidity. Recently, vertical transmission of Oropouche virus was reported, as infection in women was linked temporally to miscarriages, fetal microcephaly and congenital anomalies.
- Pyroptosis
-
A type of inflammatory cell death triggered by the formation of pores in the cell membrane by gasdermin protein family members. This cell death occurs after activation of the inflammasome, which is triggered by infection or other insults to the cell. Pore formation at the cell membrane facilitates the release of inflammatory cytokines such as IL-1β and IL-18 into the extracellular space, which act as danger signals (damage-associated molecular patterns (DAMPs)) for adjacent cells, further amplifying the inflammatory response.
- Teratogenic
-
Refers to a molecule, microorganism or agent that can cause congenital abnormalities in the context of exposure to the fetus during pregnancy. The relative deleterious effects of a teratogen depend on the agent, dose, duration and gestational stage at which the exposure occurs. Common teratogenic agents include infectious agents (viruses, bacteria, parasites), physical agents (radiation), chemicals (mercury, herbicides), prescription or recreational drugs and ingested alcohol.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alippe, Y., Hatterschide, J., Coyne, C.B. et al. Innate immune responses to pathogens at the maternal–fetal interface. Nat Rev Immunol (2025). https://doi.org/10.1038/s41577-025-01191-0
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-025-01191-0