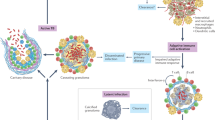
Abstract
Mycobacterium tuberculosis, the bacterium that causes tuberculosis (TB), results in more human mortality than any other single pathogen, in part because of the lack of an effective vaccine. Although T cells are essential for immunity to TB, the mechanisms that provide protective immunity are poorly understood. In this Review, we describe current gaps in our knowledge about T cell-mediated immune responses to M. tuberculosis and discuss how recent technologies, including multiphoton intravital microscopy, spatial multiomics and high-resolution in vivo analyses of cell–cell interactions, may be used to gain insights that can inform the design of T cell-targeted TB vaccines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Darrah, P. A. et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 577, 95–102 (2020).
Dijkman, K. et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 25, 255–262 (2019).
Hansen, S. G. et al. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat. Med. 24, 130–143 (2018).
Dijkman, K. et al. A protective, single-visit TB vaccination regimen by co-administration of a subunit vaccine with BCG. NPJ Vaccines 8, 66 (2023).
Woodworth, J. S. et al. A Mycobacterium tuberculosis-specific subunit vaccine that provides synergistic immunity upon co-administration with Bacillus Calmette-Guérin. Nat. Commun. 12, 6658 (2021).
Nemeth, J. et al. Contained Mycobacterium tuberculosis infection induces concomitant and heterologous protection. PLoS Pathog. 16, e1008655 (2020).
Coussens, A. K. et al. Classification of early tuberculosis states to guide research for improved care and prevention: an international Delphi consensus exercise. Lancet Respir. Med. 12, 484–498 (2024).
Garcia-Basteiro, A. L. et al. A global tuberculosis dictionary: unified terms and definitions for the field of tuberculosis. Lancet Glob. Health 12, e737–e739 (2024).
Cohen, S. B. et al. Alveolar macrophages provide an early Mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 24, 439–446.e434 (2018).
Pisu, D. et al. Single cell analysis of M. tuberculosis phenotype and macrophage lineages in the infected lung. J. Exp. Med. 218, e20210615 (2021).
Wolf, A. J. et al. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 179, 2509–2519 (2007). Myeloid DCs are key reservoirs for M. tuberculosis in the lungs and lymph nodes, while strategically impairing antigen presentation to CD4+ T cells, thereby promoting persistent infection.
Lee, J. et al. CD11cHi monocyte-derived macrophages are a major cellular compartment infected by Mycobacterium tuberculosis. PLoS Pathog. 16, e1008621 (2020).
Zheng, W. et al. Mycobacterium tuberculosis resides in lysosome-poor monocyte-derived lung cells during chronic infection. PLoS Pathog. 20, e1012205 (2024).
Lai, R., Williams, T., Rakib, T., Lee, J. & Behar, S. M. Heterogeneity in lung macrophage control of Mycobacterium tuberculosis is modulated by T cells. Nat. Commun. 15, 5710 (2024).
Kwan, C. K. & Ernst, J. D. HIV and tuberculosis: a deadly human syndemic. Clin. Microbiol. Rev. 24, 351–376 (2011).
Jasenosky, L. D., Scriba, T. J., Hanekom, W. A. & Goldfeld, A. E. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol. Rev. 264, 74–87 (2015).
Flynn, J. L. & Chan, J. Immune cell interactions in tuberculosis. Cell 185, 4682–4702 (2022).
Nathan, A. et al. Multimodally profiling memory T cells from a tuberculosis cohort identifies cell state associations with demographics, environment and disease. Nat. Immunol. 22, 781–793 (2021).
Gideon, H. P. et al. Multimodal profiling of lung granulomas in macaques reveals cellular correlates of tuberculosis control. Immunity 55, 827–846.e810 (2022). Advanced single-cell analyses, coupled with detailed in vivo measurements of Mtb granulomas, identified the cellular and transcriptional characteristics associated with an effective host immune response to TB in NHPs.
Bromley, J. D. et al. CD4+ T cells re-wire granuloma cellularity and regulatory networks to promote immunomodulation following Mtb reinfection. Immunity 57, 2380–2398 e2386 (2024).
Chackerian, A. A., Alt, J. M., Perera, T. V., Dascher, C. C. & Behar, S. M. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70, 4501–4509 (2002).
Wolf, A. J. et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 205, 105–115 (2008).
Miller, E. A. & Ernst, J. D. Anti-TNF immunotherapy and tuberculosis reactivation: another mechanism revealed. J. Clin. Invest. 119, 1079–1082 (2009).
Mathe, G. et al. Intravenous B.C.G. in monkeys and man. Lancet 1, 92 (1976).
Crispen, R. G. Immunotherapy with intravenous B.C.G. Lancet 2, 56 (1974).
Davis, J. M. & Ramakrishnan, L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49 (2009). Prompted reconsideration of the dogma that granulomas simply contain mycobacterial infection by demonstrating the role of ongoing cell recruitment in enabling cell-to-cell spread and progression of infection.
Cronan, M. R. et al. A non-canonical type 2 immune response coordinates tuberculous granuloma formation and epithelialization. Cell 184, 1757–1774 e1714 (2021).
Cohen, S. B., Gern, B. H. & Urdahl, K. B. The tuberculous granuloma and preexisting immunity. Annu. Rev. Immunol. 40, 589–614 (2022).
Sawyer, A. J. et al. Spatial mapping reveals granuloma diversity and histopathological superstructure in human tuberculosis. J. Exp. Med. 220, e20221392 (2023).
Kauffman, K. D. et al. Defective positioning in granulomas but not lung-homing limits CD4 T-cell interactions with Mycobacterium tuberculosis-infected macrophages in rhesus macaques. Mucosal Immunol. 11, 462–473 (2018).
Carow, B. et al. Spatial and temporal localization of immune transcripts defines hallmarks and diversity in the tuberculosis granuloma. Nat. Commun. 10, 1823 (2019).
McCaffrey, E. F. et al. The immunoregulatory landscape of human tuberculosis granulomas. Nat. Immunol. 23, 318–329 (2022). TB granulomas of individuals with active TB were mapped using multiplexed ion beam imaging by time of flight, identifying three spatial modules including the myeloid core enriched with IDO1 and PD-L1, lymphocytic cuff and stromal compartment revealing novel functional–spatial relationships.
Esaulova, E. et al. The immune landscape in tuberculosis reveals populations linked to disease and latency. Cell Host Microbe 29, 165–178.e168 (2021).
Magoulopoulou, A. et al. Spatial resolution of Mycobacterium tuberculosis bacteria and their surrounding immune environments based on selected key transcripts in mouse lungs. Front. Immunol. 13, 876321 (2022).
Ernst, J. D., Cornelius, A., Desvignes, L., Tavs, J. & Norris, B. A. Limited antimycobacterial efficacy of epitope peptide administration despite enhanced antigen-specific CD4 T cell activation. J. Infect. Dis. https://doi.org/10.1093/infdis/jiy142 (2018).
Szabo, P. A., Miron, M. & Farber, D. L. Location, location, location: tissue resident memory T cells in mice and humans. Sci. Immunol. 4, eaas9673 (2019).
Srivastava, S. & Ernst, J. D. Cutting edge: direct recognition of infected cells by CD4 T cells is required for control of intracellular Mycobacterium tuberculosis in vivo. J. Immunol. 191, 1016–1020 (2013). Direct recognition of infected cells by CD4+ T cells is essential for controlling intracellular Mtb, as long-range cytokine diffusion alone is insufficient to reduce bacterial burden, highlighting a critical mechanism of host defence against TB.
Muller, A. J. et al. CD4+ T cells rely on a cytokine gradient to control intracellular pathogens beyond sites of antigen presentation. Immunity 37, 147–157 (2012).
Lin, K. Y. et al. Ectopic expression of vascular cell adhesion molecule-1 as a new mechanism for tumor immune evasion. Cancer Res. 67, 1832–1841 (2007).
Tian, C. et al. Motility and tumor infiltration are key aspects of invariant natural killer T cell anti-tumor function. Nat. Commun. 15, 1213 (2024).
Nunes-Alves, C. et al. In search of a new paradigm for protective immunity to TB. Nat. Rev. Microbiol. 12, 289–299 (2014).
Winchell, C. G. et al. CD8+ lymphocytes are critical for early control of tuberculosis in macaques. J. Exp. Med. 220, e20230707 (2023). The study investigates the role of innate and conventional CD8 immunity in TB, revealing that CD8αα+ lymphocytes have a key role in the early suppression of Mtb growth in macaque lungs.
Cai, Y. et al. Single-cell immune profiling reveals functional diversity of T cells in tuberculous pleural effusion. J. Exp. Med. 219, e20211777 (2022).
Schurr, E. et al. Mycobacterium tuberculosis resisters despite HIV exhibit activated T cells and macrophages in their pulmonary alveoli. J. Clin. Invest. 135, e188016 (2025). Poly-cytotoxic T cells and alveolar macrophages contribute to natural resistance to Mtb infection in individuals with HIV.
Simonson, A. W. et al. CD4 T cells and CD8α+ lymphocytes are necessary for intravenous BCG-induced protection against tuberculosis in macaques. J. Exp. Med. 222, e20241571 (2025). This article presents evidence that CD8+ and CD4+ T cells have a crucial role in mediating protection in NHPs vaccinated with intravenous BCG.
Patankar, Y. R. et al. Limited recognition of Mycobacterium tuberculosis-infected macrophages by polyclonal CD4 and CD8 T cells from the lungs of infected mice. Mucosal Immunol. 13, 140–148 (2020).
Carpenter, S. M., Nunes-Alves, C., Booty, M. G., Way, S. S. & Behar, S. M. A higher activation threshold of memory CD8+ T cells has a fitness cost that is modified by TCR affinity during tuberculosis. PLoS Pathog. 12, e1005380 (2016).
Billeskov, R., Vingsbo-Lundberg, C., Andersen, P. & Dietrich, J. Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J. Immunol. 179, 3973–3981 (2007).
Yang, J. D. et al. Mycobacterium tuberculosis-specific CD4+ and CD8+ T cells differ in their capacity to recognize infected macrophages. PLoS Pathog. 14, e1007060 (2018).
Sutiwisesak, R. et al. A natural polymorphism of Mycobacterium tuberculosis in the esxH gene disrupts immunodomination by the TB10.4-specific CD8 T cell response. PLoS Pathog. 16, e1009000 (2020).
Nyendak, M. et al. Adenovirally-induced polyfunctional T cells do not necessarily recognize the infected target: lessons from a phase I trial of the AERAS-402 vaccine. Sci. Rep. 6, 36355 (2016).
Behar, S. M., Martin, C. J., Nunes-Alves, C., Divangahi, M. & Remold, H. G. Lipids, apoptosis, and cross-presentation: links in the chain of host defense against Mycobacterium tuberculosis. Microbes Infect. 13, 749–756 (2011).
Athman, J. J. et al. Mycobacterium tuberculosis membrane vesicles inhibit T cell activation. J. Immunol. 198, 2028–2037 (2017).
Caouaille, M., Hudrisier, D. & Neyrolles, O. Mycobacterial d-serine impairs TB control. Nat. Immunol. 25, 1129–1130 (2024).
Cheng, H. et al. Mycobacterium tuberculosis produces d-serine under hypoxia to limit CD8+ T cell-dependent immunity in mice. Nat. Microbiol. 9, 1856–1872 (2024).
Mahon, R. N. et al. Mycobacterium tuberculosis cell wall glycolipids directly inhibit CD4+ T-cell activation by interfering with proximal T-cell-receptor signaling. Infect. Immun. 77, 4574–4583 (2009).
Mahon, R. N. et al. Mycobacterium tuberculosis ManLAM inhibits T-cell-receptor signaling by interference with ZAP-70, Lck and LAT phosphorylation. Cell Immunol. 275, 98–105 (2012).
Mwebaza, I. et al. Impact of Mycobacterium tuberculosis glycolipids on the CD4+ T cell-macrophage immunological synapse. J. Immunol. 211, 1385–1396 (2023).
Sande, O. J. et al. Mannose-capped lipoarabinomannan from Mycobacterium tuberculosis induces CD4+ T cell anergy via GRAIL. J. Immunol. 196, 691–702 (2016).
Collins, J. M. et al. Tryptophan catabolism reflects disease activity in human tuberculosis. JCI Insight 5, e137131 (2020).
Gautam, U. S. et al. In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 115, E62–E71 (2018). Inhibiting IDO activity in tuberculomas in macaques induces the reorganization of the granulomata, with T cells otherwise present in the peripheral region of lesions being able to gain greater access to the core region. This can potentially improve TB control by enhancing immune-mediated control and reducing bacterial burden and pathology.
Desvignes, L. & Ernst, J. D. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity 31, 974–985 (2009).
Ahmed, A. & Vyakarnam, A. Emerging patterns of regulatory T cell function in tuberculosis. Clin. Exp. Immunol. 202, 273–287 (2020).
Kotov, D. I. et al. Early cellular mechanisms of type I interferon-driven susceptibility to tuberculosis. Cell 186, 5536–53.e22 (2023). Plasmacytoid DCs drive Mtb pathogenesis by producing type I interferons that impair interstitial macrophage responses to IFNγ, promoting bacterial replication, neutrophil recruitment and active tuberculosis disease.
Ji, D. X. et al. Type I interferon-driven susceptibility to Mycobacterium tuberculosis is mediated by IL-1Ra. Nat. Microbiol. 4, 2128–2135 (2019).
Berry, M. P. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010).
Moreira-Teixeira, L., Mayer-Barber, K., Sher, A. & O’Garra, A. Type I interferons in tuberculosis: foe and occasionally friend. J. Exp. Med. 215, 1273–1285 (2018).
Lazar-Molnar, E. et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc. Natl Acad. Sci. USA 107, 13402–13407 (2010).
Kauffman, K. D. et al. PD-1 blockade exacerbates Mycobacterium tuberculosis infection in rhesus macaques. Sci. Immunol. 6, eabf3861 (2021).
Jayaraman, P. et al. TIM3 mediates T cell exhaustion during Mycobacterium tuberculosis infection. PLoS Pathog. 12, e1005490 (2016).
Zhu, C. et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat. Commun. 6, 6072 (2015).
Torrado, E. et al. Interleukin 27R regulates CD4+ T cell phenotype and impacts protective immunity during Mycobacterium tuberculosis infection. J. Exp. Med. 212, 1449–1463 (2015).
Panda, S. et al. Identification of differentially recognized T cell epitopes in the spectrum of tuberculosis infection. Nat. Commun. 15, 765 (2024).
Lindestam Arlehamn, C. S. et al. A quantitative analysis of complexity of human pathogen-specific CD4 T cell responses in healthy M. tuberculosis infected South Africans. PLoS Pathog. 12, e1005760 (2016).
Lewinsohn, D. M. et al. Human Mycobacterium tuberculosis CD8 T cell antigens/epitopes identified by a proteomic peptide library. PLoS ONE 8, e67016 (2013).
Samstein, M. et al. Essential yet limited role for CCR2+ inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. eLife 2, e01086 (2013).
Srivastava, S. & Ernst, J. D. Cell-to-cell transfer of M. tuberculosis antigens optimizes CD4 T cell priming. Cell Host Microbe 15, 741–752 (2014). During Mtb infection, inefficient antigen presentation by infected migratory DCs is bypassed through the release and transfer of bacterial antigens to uninfected resident lymph node DCs, enabling optimal CD4+ T cell priming and enhancing immune control of TB.
Delamarre, L., Pack, M., Chang, H., Mellman, I. & Trombetta, E. S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307, 1630–1634 (2005).
Commandeur, S. et al. An unbiased genome-wide Mycobacterium tuberculosis gene expression approach to discover antigens targeted by human T cells expressed during pulmonary infection. J. Immunol. 190, 1659–1671 (2013).
Bold, T. D., Banaei, N., Wolf, A. J. & Ernst, J. D. Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. 7, e1002063 (2011).
Rogerson, B. J. et al. Expression levels of Mycobacterium tuberculosis antigen-encoding genes versus production levels of antigen-specific T cells during stationary level lung infection in mice. Immunology 118, 195–201 (2006).
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Rothchild, A. C. et al. Alveolar macrophages generate a noncanonical NRF2-driven transcriptional response to Mycobacterium tuberculosis in vivo. Sci. Immunol. 4, eaaw6693 (2019).
Eisenbarth, S. C. Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 19, 89–103 (2019).
Shah, J. A., Lindestam Arlehamn, C. S., Horne, D. J., Sette, A. & Hawn, T. R. Nontuberculous mycobacteria and heterologous immunity to tuberculosis. J. Infect. Dis. 220, 1091–1098 (2019).
Simeone, R. et al. Cytosolic access of Mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog. 11, e1004650 (2015).
Divangahi, M. et al. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat. Immunol. 10, 899–906 (2009).
Woodworth, J. S., Fortune, S. M. & Behar, S. M. Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect. Immun. 76, 4199–4205 (2008).
Woodworth, J. S. & Behar, S. M. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit. Rev. Immunol. 26, 317–352 (2006).
Lewinsohn, D. A. et al. Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-B. PLoS Pathog. 3, 1240–1249 (2007).
Lewinsohn, D. M. et al. Secreted proteins from Mycobacterium tuberculosis gain access to the cytosolic MHC class-I antigen-processing pathway. J. Immunol. 177, 437–442 (2006).
Tobian, A. A., Canaday, D. H., Boom, W. H. & Harding, C. V. Bacterial heat shock proteins promote CD91-dependent class I MHC cross-presentation of chaperoned peptide to CD8+ T cells by cytosolic mechanisms in dendritic cells versus vacuolar mechanisms in macrophages. J. Immunol. 172, 5277–5286 (2004).
Grotzke, J. E., Siler, A. C., Lewinsohn, D. A. & Lewinsohn, D. M. Secreted immunodominant Mycobacterium tuberculosis antigens are processed by the cytosolic pathway. J. Immunol. 185, 4336–4343 (2010).
Grotzke, J. E. et al. The Mycobacterium tuberculosis phagosome is a HLA-I processing competent organelle. PLoS Pathog. 5, e1000374 (2009).
Mazzaccaro, R. J. et al. Major histocompatibility class I presentation of soluble antigen facilitated by Mycobacterium tuberculosis infection. Proc. Natl Acad. Sci. USA 93, 11786–11791 (1996).
Mott, D. et al. High bacillary burden and the ESX-1 type VII secretion system promote MHC class I presentation by Mycobacterium tuberculosis-infected macrophages to CD8 T cells. J. Immunol. 210, 1531–1542 (2023).
Schaible, U. E. et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 9, 1039–1046 (2003).
Tzelepis, F. et al. Annexin1 regulates DC efferocytosis and cross-presentation during Mycobacterium tuberculosis infection. J. Clin. Invest. 125, 752–768 (2015).
Smith, V. L., Cheng, Y., Bryant, B. R. & Schorey, J. S. Exosomes function in antigen presentation during an in vivo Mycobacterium tuberculosis infection. Sci. Rep. 7, 43578 (2017).
Baptista, A. P. et al. The chemoattractant receptor Ebi2 drives intranodal naive CD4+ T cell peripheralization to promote effective adaptive immunity. Immunity 50, 1188–1201 e1186 (2019).
Kapoor, V. N. et al. Gremlin 1+ fibroblastic niche maintains dendritic cell homeostasis in lymphoid tissues. Nat. Immunol. 22, 571–585 (2021).
Leal, J. M. et al. Innate cell microenvironments in lymph nodes shape the generation of T cell responses during type I inflammation. Sci. Immunol. 6, eabb9435 (2021).
Daniel, L. et al. Stromal structure remodeling by B lymphocytes limits T cell activation in lymph nodes of Mycobacterium tuberculosis-infected mice. J. Clin. Invest. 132, e157873 (2022).
Musvosvi, M. et al. T cell receptor repertoires associated with control and disease progression following Mycobacterium tuberculosis infection. Nat. Med. 29, 258–269 (2023). Using both deep TCR sequencing and genome-wide antigen screen, the authors identified peptides targeted by T cell similarity groups enriched either in controllers or in progressors.
Chugh, S., Bahal, R. K., Dhiman, R. & Singh, R. Antigen identification strategies and preclinical evaluation models for advancing tuberculosis vaccine development. NPJ Vaccines 9, 57 (2024).
Comas, I. et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Genet. 42, 498–503 (2010). The hyperconservation of human T cell epitopes in Mtb suggests strong purifying selection, indicating that immune recognition may have a role in the persistence and evolutionary success of the pathogen.
Ogongo, P. et al. Rare variable M. tuberculosis antigens induce predominant Th17 responses in human infection. Preprint at bioRxiv https://doi.org/10.1101/2024.03.05.583634 (2024). Rare variable Mtb antigens preferentially induce IL-17-producing TH17 cells, suggesting their potential as vaccine targets to enhance protective immunity in individuals already exposed to TB.
Lindestam Arlehamn, C. S., Lewinsohn, D., Sette, A. & Lewinsohn, D. Antigens for CD4 and CD8 T cells in tuberculosis. Cold Spring Harb. Perspect. Med. 4, a018465 (2014).
Sakai, S. et al. CD4 T cell-derived IFN-gamma plays a minimal role in control of pulmonary Mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog. 12, e1005667 (2016).
Coppola, M. et al. New genome-wide algorithm identifies novel in-vivo expressed Mycobacterium Tuberculosis antigens inducing human T-cell responses with classical and unconventional cytokine profiles. Sci. Rep. 6, 37793 (2016).
Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8, 369–377 (2007). Vaccination induces IL-17-producing CD4+ T cells that, upon Mtb infection, drive chemokine-mediated recruitment of IFNγ-producing CD4+ T cells to the lung, accelerating bacterial control and highlighting IL-23 as a key regulator of protective immunity.
Scriba, T. J. et al. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog. 13, e1006687 (2017).
Moguche, A. O. et al. Antigen availability shapes T cell differentiation and function during tuberculosis. Cell Host Microbe 21, 695–706.e695 (2017). Effective Mtb vaccine design requires tailored strategies, as Ag85B-specific CD4+ T cells are limited by reduced antigen expression during persistent infection, and ESAT-6-specific T cells become functionally exhausted due to chronic stimulation, restricting their ability to control TB.
Coscolla, M. et al. M. tuberculosis T cell epitope analysis reveals paucity of antigenic variation and identifies rare variable TB antigens. Cell Host Microbe https://doi.org/10.1016/j.chom.2015.10.008 (2015).
Coppola, M. et al. In-vivo expressed Mycobacterium tuberculosis antigens recognised in three mouse strains after infection and BCG vaccination. NPJ Vaccines 6, 81 (2021).
Nunes-Alves, C. et al. Human and murine clonal CD8+ T cell expansions arise during tuberculosis because of TCR selection. PLoS Pathog. 11, e1004849 (2015).
Carpenter, S. M., Yang, J. D., Lee, J., Barreira-Silva, P. & Behar, S. M. Vaccine-elicited memory CD4+ T cell expansion is impaired in the lungs during tuberculosis. PLoS Pathog. 13, e1006704 (2017). The transient protection of TB vaccines is driven by the inability of memory CD4+ T cells to sustain expansion in the lung, as their initial proliferation and recruitment curb early Mtb growth but fail to maintain long-term immunity.
Bhattacharyya, N. D. et al. TCR affinity controls the dynamics but not the functional specification of the antimycobacterial CD4+ T cell response. J. Immunol. 206, 2875–2887 (2021).
Ogongo, P. & Ernst, J. D. Finding antigens for TB vaccines: the good, the bad and the useless. Nat. Med. 29, 35–36 (2023).
Alon, R. et al. Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19. Nat. Rev. Immunol. 21, 49–64 (2021).
Hoft, S. G. et al. The rate of CD4 T cell entry into the lungs during Mycobacterium tuberculosis infection is determined by partial and opposing effects of multiple chemokine receptors. Infect. Immun. 87, e00841–18 (2019).
Santiago-Carvalho, I. et al. T cell-specific P2RX7 favors lung parenchymal CD4+ T cell accumulation in response to severe lung infections. Cell Rep. 42, 113448 (2023). Extracellular ATP released by damaged cells is sensed by CD4+ T cells expressing P2RX7 receptor and critical to induce tissue CD4+ T cell accumulation through upregulation of the chemokine receptor CXCR3.
Egen, J. G. et al. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity 34, 807–819 (2011). Intravital imaging in BCG-infected liver show that few T cells are arrested within the granuloma and stably interact with APC, indicating that limited antigen presentation in mycobacterial granulomas leads to a muted T cell response, using only a fraction of the host’s potential effector capacity during chronic infection.
Stark, R. et al. TRM maintenance is regulated by tissue damage via P2RX7. Sci. Immunol. 3, eaau1022 (2018). Extracellular ATP and NAD+ enhanced TRM cell death via P2RX7, whereas TCR activation downregulated P2RX7, making TRM cells resistant to NAD-induced death. Tissue damage regulates TRM cells through P2RX7, favouring antigen-specific TRM cell persistence.
Gern, B. H. et al. TGFβ restricts expansion, survival, and function of T cells within the tuberculous granuloma. Cell Host Microbe 29, 594–606.e596 (2021).
Kang, T. G. et al. Viral coinfection promotes tuberculosis immunopathogenesis by type I IFN signaling-dependent impediment of Th1 cell pulmonary influx. Nat. Commun. 13, 3155 (2022).
Potter, E. L. et al. Measurement of leukocyte trafficking kinetics in macaques by serial intravascular staining. Sci. Transl. Med. 13, eabb4582 (2021).
Takamura, S. et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J. Exp. Med. 213, 3057–3073 (2016).
Asrir, A. et al. Tumor-associated high endothelial venules mediate lymphocyte entry into tumors and predict response to PD-1 plus CTLA-4 combination immunotherapy. Cancer Cell 40, 318–334.e319 (2022). Tumour-associated HEVs serve as key entry points for lymphocytes into tumours, enhancing immune checkpoint blockade efficacy by promoting infiltration of stem-like CD8+ T cells, with their presence correlating with better responses and survival in patients with metastatic melanoma.
Blanchard, L. & Girard, J. P. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis 24, 719–753 (2021).
Carow, B. et al. Immune mapping of human tuberculosis and sarcoidosis lung granulomas. Front. Immunol. 14, 1332733 (2023).
Kahnert, A. et al. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J. Infect. Dis. 195, 46–54 (2007).
Pally, D. & Naba, A. Extracellular matrix dynamics: a key regulator of cell migration across length-scales and systems. Curr. Opin. Cell Biol. 86, 102309 (2024).
Overstreet, M. G. et al. Inflammation-induced interstitial migration of effector CD4+ T cells is dependent on integrin alphaV. Nat. Immunol. 14, 949–958 (2013).
Wilson, E. H. et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity 30, 300–311 (2009).
Salmon, H. et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 122, 899–910 (2012).
Al Shammari, B. et al. The extracellular matrix regulates granuloma necrosis in tuberculosis. J. Infect. Dis. 212, 463–473 (2015).
Bromley, S. K., Peterson, D. A., Gunn, M. D. & Dustin, M. L. Cutting edge: hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J. Immunol. 165, 15–19 (2000). This study provided insight into the relationships between chemokine receptor and TCR signalling in determining trafficking and positioning of T cells in immunity.
Li, J. et al. Mycobacterium tuberculosis-specific memory T cells in bronchoalveolar lavage of patients with pulmonary tuberculosis. Cytokine 171, 156374 (2023).
Yang, Q. et al. Cutting edge: characterization of human tissue-resident memory T cells at different infection sites in patients with tuberculosis. J. Immunol. 204, 2331–2336 (2020).
Morrison, H. & McShane, H. Local pulmonary immunological biomarkers in tuberculosis. Front. Immunol. 12, 640916 (2021).
Hunter, R. L. Tuberculosis as a three-act play: a new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis 97, 8–17 (2016).
Jameson, S. C. The naming of memory T-cell subsets. Cold Spring Harb. Perspect. Biol. 13, a037788 (2021).
Christo, S. N., Park, S. L., Mueller, S. N. & Mackay, L. K. The multifaceted role of tissue-resident memory T cells. Annu. Rev. Immunol. 42, 317–345 (2024).
Mueller, S. N., Gebhardt, T., Carbone, F. R. & Heath, W. R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31, 137–161 (2013).
Amezcua Vesely, M. C. et al. Effector TH17 cells give rise to long-lived TRM cells that are essential for an immediate response against bacterial infection. Cell 178, 1176–1188.e1115 (2019).
Haddadi, S. et al. Expression and role of VLA-1 in resident memory CD8 T cell responses to respiratory mucosal viral-vectored immunization against tuberculosis. Sci. Rep. 7, 9525 (2017).
Perdomo, C. et al. Mucosal BCG vaccination induces protective lung-resident memory T cell populations against tuberculosis. MBio 7, e01686–16 (2016).
Hu, Z. et al. Sendai virus mucosal vaccination establishes lung-resident memory CD8 T cell immunity and boosts BCG-primed protection against TB in mice. Mol. Ther. 25, 1222–1233 (2017).
Dijkman, K. et al. Pulmonary MTBVAC vaccination induces immune signatures previously correlated with prevention of tuberculosis infection. Cell Rep. Med. 2, 100187 (2021).
Palendira, U., Bean, A. G., Feng, C. G. & Britton, W. J. Lymphocyte recruitment and protective efficacy against pulmonary mycobacterial infection are independent of the route of prior Mycobacterium bovis BCG immunization. Infect. Immun. 70, 1410–1416 (2002).
Ganchua, S. K. et al. Antibiotic treatment modestly reduces protection against Mycobacterium tuberculosis reinfection in macaques. Infect. Immun. 92, e0053523 (2024).
Stolley, J. M. et al. Depleting CD103+ resident memory T cells in vivo reveals immunostimulatory functions in oral mucosa. J. Exp. Med. 220, e20221853 (2023).
Behr, F. M. et al. Tissue-resident memory CD8+ T cells shape local and systemic secondary T cell responses. Nat. Immunol. 21, 1070–1081 (2020).
Florido, M. et al. Pulmonary immunization with a recombinant influenza A virus vaccine induces lung-resident CD4+ memory T cells that are associated with protection against tuberculosis. Mucosal Immunol. 11, 1743–1752 (2018).
Turner, D. L. et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 7, 501–510 (2014).
Ogongo, P. et al. Tissue-resident-like CD4+ T cells secreting IL-17 control Mycobacterium tuberculosis in the human lung. J. Clin. Invest. 131, e142014 (2021).
Alexandrov, T., Saez-Rodriguez, J. & Saka, S. K. Enablers and challenges of spatial omics, a melting pot of technologies. Mol. Syst. Biol. 19, e10571 (2023).
Breart, B., Lemaitre, F., Celli, S. & Bousso, P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J. Clin. Invest. 118, 1390–1397 (2008).
Filipe-Santos, O. et al. A dynamic map of antigen recognition by CD4 T cells at the site of Leishmania major infection. Cell Host Microbe 6, 23–33 (2009).
Ashouri, J. F. & Weiss, A. Endogenous Nur77 is a specific indicator of antigen receptor signaling in human T and B cells. J. Immunol. 198, 657–668 (2017).
Moran, A. E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Formaglio, P. et al. Nitric oxide controls proliferation of Leishmania major by inhibiting the recruitment of permissive host cells. Immunity 54, 2724–2739 e2710 (2021).
Halle, S. et al. In vivo killing capacity of cytotoxic T cells is limited and involves dynamic interactions and T cell cooperativity. Immunity 44, 233–245 (2016).
Looney, M. R. & Headley, M. B. Live imaging of the pulmonary immune environment. Cell Immunol. 350, 103862 (2020). The review summarizes how advances in intravital microscopy have enhanced our understanding of lung function and immunity in steady state and diseases by visualizing immune dynamics in real-time.
Ueki, H., Wang, I. H., Zhao, D., Gunzer, M. & Kawaoka, Y. Multicolor two-photon imaging of in vivo cellular pathophysiology upon influenza virus infection using the two-photon IMPRESS. Nat. Protoc. 15, 1041–1065 (2020).
Barlerin, D. et al. Biosafety level 3 setup for multiphoton microscopy in vivo. Sci. Rep. 7, 571 (2017).
Gazit, R. et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 7, 517–523 (2006).
Ovchinnikov, D. A. et al. Expression of Gal4-dependent transgenes in cells of the mononuclear phagocyte system labeled with enhanced cyan fluorescent protein using Csf1r-Gal4VP16/UAS-ECFP double-transgenic mice. J. Leukoc. Biol. 83, 430–433 (2008).
Lindquist, R. L. et al. Visualizing dendritic cell networks in vivo. Nat. Immunol. 5, 1243–1250 (2004).
Boulch, M. et al. A cross-talk between CAR T cell subsets and the tumor microenvironment is essential for sustained cytotoxic activity. Sci. Immunol. 6, eabd4344 (2021).
Boulch, M., Grandjean, C. L., Cazaux, M. & Bousso, P. Tumor immunosurveillance and immunotherapies: a fresh look from intravital imaging. Trends Immunol. 40, 1022–1034 (2019).
Grandjean, C. L., Garcia, Z., Lemaitre, F., Breart, B. & Bousso, P. Imaging the mechanisms of anti-CD20 therapy in vivo uncovers spatiotemporal bottlenecks in antibody-dependent phagocytosis. Sci. Adv. 7, eabd6167 (2021).
Wu, J. et al. A highly polarized TH2 bladder response to infection promotes epithelial repair at the expense of preventing new infections. Nat. Immunol. 21, 671–683 (2020).
Hoekstra, M. E., Dijkgraaf, F. E., Schumacher, T. N. & Rohr, J. C. Assessing T lymphocyte function and differentiation by genetically encoded reporter systems. Trends Immunol. 36, 392–400 (2015).
Chitirala, P. et al. Studying the biology of cytotoxic T lymphocytes in vivo with a fluorescent granzyme B-mTFP knock-in mouse. eLife 9, e58065 (2020).
Vorobjev, I. A. & Barteneva, N. S. Multi-parametric imaging of cell heterogeneity in apoptosis analysis. Methods 112, 105–123 (2017).
Fukumura, D., Duda, D. G., Munn, L. L. & Jain, R. K. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 17, 206–225 (2010).
McCarthy, C. E., White, J. M., Viola, N. T. & Gibson, H. M. In vivo imaging technologies to monitor the immune system. Front. Immunol. 11, 1067 (2020).
MacGilvary, N. J. & Tan, S. Fluorescent Mycobacterium tuberculosis reporters: illuminating host-pathogen interactions. Pathog. Dis. 76, fty017 (2018).
Huang, L., Nazarova, E. V., Tan, S., Liu, Y. & Russell, D. G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. https://doi.org/10.1084/jem.20172020 (2018).
Tan, S., Sukumar, N., Abramovitch, R. B., Parish, T. & Russell, D. G. Mycobacterium tuberculosis responds to chloride and pH as synergistic cues to the immune status of its host cell. PLoS Pathog. 9, e1003282 (2013).
Liu, Z. et al. Detecting tumor antigen-specific T cells via interaction-dependent fucosyl-biotinylation. Cell 183, 1117–1133.e19 (2020).
Qiu, S. et al. Use of intercellular proximity labeling to quantify and decipher cell-cell interactions directed by diversified molecular pairs. Sci. Adv. 8, eadd2337 (2022).
Pasqual, G. et al. Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling. Nature 553, 496–500 (2018).
Nakandakari-Higa, S. et al. Universal recording of immune cell interactions in vivo. Nature 627, 399–406 (2024). A new genetically encoded method for proximity labelling to identify cell–cell interactions in vivo, such as T cell–APC interactions.
Kiepiela, P. et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13, 46–53 (2007).
Ranasinghe, S. et al. HIV-specific CD4 T cell responses to different viral proteins have discordant associations with viral load and clinical outcome. J. Virol. 86, 277–283 (2012).
Coppola, M. & Ottenhoff, T. H. Genome wide approaches discover novel Mycobacterium tuberculosis antigens as correlates of infection, disease, immunity and targets for vaccination. Semin. Immunol. 39, 88–101 (2018).
Nair, S. K. et al. High-throughput identification and dendritic cell-based functional validation of MHC class I-restricted Mycobacterium tuberculosis epitopes. Sci. Rep. 4, 4632 (2014).
Lichti, C. F., Vigneron, N., Clauser, K. R., Van den Eynde, B. J. & Bassani-Sternberg, M. Navigating critical challenges associated with immunopeptidomics-based detection of proteasomal spliced peptide candidates. Cancer Immunol. Res. 10, 275–284 (2022).
Gulden, P. H. et al. A listeria monocytogenes pentapeptide is presented to cytolytic T lymphocytes by the H2-M3 MHC class Ib molecule. Immunity 5, 73–79 (1996).
Purcell, A. W., Ramarathinam, S. H. & Ternette, N. Mass spectrometry-based identification of MHC-bound peptides for immunopeptidomics. Nat. Protoc. 14, 1687–1707 (2019).
Shao, W. et al. The SysteMHC atlas project. Nucleic Acids Res. 46, D1237–D1247 (2018).
Bettencourt, P. et al. Identification of antigens presented by MHC for vaccines against tuberculosis. NPJ Vaccines 5, 2 (2020).
Leddy, O., White, F. M. & Bryson, B. D. Immunopeptidomics reveals determinants of Mycobacterium tuberculosis antigen presentation on MHC class I. eLife 12, e84070 (2023).
Cayabyab, M. J., Qin, L., Kashino, S. S., Izzo, A. & Campos-Neto, A. An unbiased peptide-wide discovery approach to select Mycobacterium tuberculosis antigens that target CD8+ T cell response during infection. Vaccine 31, 4834–4840 (2013).
Baert, L. et al. Induced pluripotent stem cell-derived human macrophages as an infection model for Leishmania donovani. PLoS Negl. Trop. Dis. 18, e0011559 (2024).
Higaki, K. et al. Generation of HIV-resistant macrophages from IPSCs by using transcriptional gene silencing and promoter-targeted RNA. Mol. Ther. Nucleic Acids 12, 793–804 (2018).
Hong, D. et al. Human-induced pluripotent stem cell-derived macrophages and their immunological function in response to tuberculosis infection. Stem Cell Res. Ther. 9, 49 (2018).
Nielsen, M., Ternette, N. & Barra, C. The interdependence of machine learning and LC–MS approaches for an unbiased understanding of the cellular immunopeptidome. Expert. Rev. Proteom. 19, 77–88 (2022).
Huang, S. et al. CD1 lipidomes reveal lipid-binding motifs and size-based antigen-display mechanisms. Cell 186, 4583–4596 e4513 (2023).
Beckman, E. M. et al. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 372, 691–694 (1994).
Moody, D. B. et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404, 884–888 (2000).
Huang, H., Wang, C., Rubelt, F., Scriba, T. J. & Davis, M. M. Analyzing the Mycobacterium tuberculosis immune response by T-cell receptor clustering with GLIPH2 and genome-wide antigen screening. Nat. Biotechnol. 38, 1194–1202 (2020).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Mead, H. L. et al. Integrated, high-dimensional analysis of CD4 T cell epitope specificities and phenotypes reveals unexpected diversity in the response to Mycobacterium tuberculosis. Preprint at bioRxiv https://doi.org/10.1101/2024.11.05.622086 (2024).
Ogongo, P. et al. Differential skewing of donor-unrestricted and γδ T cell repertoires in tuberculosis-infected human lungs. J. Clin. Investig. 130, 214–230 (2020).
Kramnik, I., Dietrich, W. F., Demant, P. & Bloom, B. R. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 97, 8560–8565 (2000).
Churchill, G. A. et al. The collaborative cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 36, 1133–1137 (2004).
Churchill, G. A., Gatti, D. M., Munger, S. C. & Svenson, K. L. The diversity outbred mouse population. Mamm. Genome 23, 713–718 (2012).
Niazi, M. K. et al. Lung necrosis and neutrophils reflect common pathways of susceptibility to Mycobacterium tuberculosis in genetically diverse, immune-competent mice. Dis. Model. Mech. 8, 1141–1153 (2015).
Smith, C. M. et al. Host–pathogen genetic interactions underlie tuberculosis susceptibility in genetically diverse mice. eLife 11, e74419 (2022).
Lai, R. et al. Host genetic background is a barrier to broadly effective vaccine-mediated protection against tuberculosis. J. Clin. Invest. 133, e167762 (2023).
Wu, R., Velickovic, M. & Burnum-Johnson, K. E. From single cell to spatial multi-omics: unveiling molecular mechanisms in dynamic and heterogeneous systems. Curr. Opin. Biotechnol. 89, 103174 (2024).
Larenas-Munoz, F. et al. Proteomic analysis of granulomas from cattle and pigs naturally infected with Mycobacterium tuberculosis complex by MALDI imaging. Front. Immunol. 15, 1369278 (2024).
Greco, F. et al. Lipids associated with atherosclerotic plaque instability revealed by mass spectrometry imaging of human carotid arteries. Atherosclerosis 397, 118555 (2024).
Shariatgorji, R. et al. Spatial visualization of comprehensive brain neurotransmitter systems and neuroactive substances by selective in situ chemical derivatization mass spectrometry imaging. Nat. Protoc. 16, 3298–3321 (2021).
Rajbhandari, P., Neelakantan, T. V., Hosny, N. & Stockwell, B. R. Spatial pharmacology using mass spectrometry imaging. Trends Pharmacol. Sci. 45, 67–80 (2024).
Blanc, L. et al. High-resolution mapping of fluoroquinolones in TB rabbit lesions reveals specific distribution in immune cell types. eLife 7, e41115 (2018).
Moore, M. J. et al. Humanization of T cell-mediated immunity in mice. Sci. Immunol. 6, eabj4026 (2021).
Patel, S., Liu, W., K, R., McCormick, C. & Fan, Y. Engineering immune organoids to regenerate host immune system. Curr. Opin. Genet. Dev. 89, 102276 (2024).
Wagar, L. E. et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 27, 125–135 (2021).
Ramachandra, L., Noss, E., Boom, W. H. & Harding, C. V. Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomal formation of peptide-major histocompatibility complex II complexes and is inhibited by live bacilli that decrease phagosome maturation. J. Exp. Med. 194, 1421–1432 (2001).
Mehra, A. et al. Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog. 9, e1003734 (2013).
Portal-Celhay, C. et al. Mycobacterium tuberculosis EsxH inhibits ESCRT-dependent CD4+ T-cell activation. Nat. Microbiol. 2, 16232 (2016).
Madan-Lala, R. et al. Mycobacterium tuberculosis impairs dendritic cell functions through the serine hydrolase Hip1. J. Immunol. 192, 4263–4272 (2014).
Dolasia, K., Nazar, F. & Mukhopadhyay, S. Mycobacterium tuberculosis PPE18 protein inhibits MHC class II antigen presentation and B cell response in mice. Eur. J. Immunol. 51, 603–619 (2021).
Saini, N. K. et al. Suppression of autophagy and antigen presentation by Mycobacterium tuberculosis PE_PGRS47. Nat. Microbiol. 1, 16133 (2016).
Fulton, S. A. et al. Inhibition of major histocompatibility complex II expression and antigen processing in murine alveolar macrophages by Mycobacterium bovis BCG and the 19-kilodalton mycobacterial lipoprotein. Infect. Immun. 72, 2101–2110 (2004).
Gehring, A. J. et al. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through toll-like receptor 2. Infect. Immun. 71, 4487–4497 (2003).
Pai, R. K., Convery, M., Hamilton, T. A., Boom, W. H. & Harding, C. V. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 171, 175–184 (2003).
Su, H. et al. Recombinant lipoprotein Rv1016c derived from Mycobacterium tuberculosis is a TLR-2 ligand that induces macrophages apoptosis and inhibits MHC II antigen processing. Front. Cell Infect. Microbiol. 6, 147 (2016).
Pecora, N. D., Gehring, A. J., Canaday, D. H., Boom, W. H. & Harding, C. V. Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J. Immunol. 177, 422–429 (2006).
Gehring, A. J., Dobos, K. M., Belisle, J. T., Harding, C. V. & Boom, W. H. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J. Immunol. 173, 2660–2668 (2004).
Huber, A. et al. Mycobacterial cord factor reprograms the macrophage response to IFN-gamma towards enhanced inflammation yet impaired antigen presentation and expression of GBP1. J. Immunol. 205, 1580–1592 (2020).
Srivastava, S., Grace, P. S. & Ernst, J. D. Antigen export reduces antigen presentation and limits T cell control of M. tuberculosis. Cell Host Microbe 19, 44–54 (2016).Mtb evades CD4+ T cell immunity by exporting antigens from infected DCs, diverting them from the antigen presentation pathway, a process dependent on kinesin-2, whose depletion enhances T cell activation and intracellular bacterial control.
Grace, P. S. & Ernst, J. D. Suboptimal antigen presentation contributes to virulence of Mycobacterium tuberculosis in vivo. J. Immunol. 196, 357–364 (2016).
Sreejit, G. et al. The ESAT-6 protein of Mycobacterium tuberculosis interacts with beta-2-microglobulin (β2M) affecting antigen presentation function of macrophage. PLoS Pathog. 10, e1004446 (2014).
Velmurugan, K. et al. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 3, e110 (2007).
Winau, F. et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 24, 105–117 (2006).
Singh, B. et al. Inhibition of indoleamine dioxygenase leads to better control of tuberculosis adjunctive to chemotherapy. JCI Insight 8, e163101 (2023).
Barber, D. L., Mayer-Barber, K. D., Feng, C. G., Sharpe, A. H. & Sher, A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J. Immunol. 186, 1598–1607 (2011).
Acknowledgements
This study was supported by the French National Research Agency: JCJC (grant ANR-20-CE14-0045-01) and TBVAC Horizon Europe programme (E.L.); Agence Nationale de le Recherche sur le SIDA — Maladies Infectieuses Emergentes KILL-TB ECTZ206385 (D.H.); TBVAC-HORIZON (EU), Immunotherapies for Tuberculosis and Other Mycobacterial Diseases (EU) and EXPLORE-TB (Fondation Bettencourt Schueller) (O.N.); National Institutes of Health, National Institute of Allergy and Infectious Diseases (grants R01AI172905 to S.M.B., U01AI166309, R21AI156407 and R21AI176234 to J.D.E. and contracts 75N93019C00071 to S.M.B. and 75N93024C0054 to J.D.E.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Stefan Kaufmann, Thomas Scriba, Cecilia Lindestam Arlehamn and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lefrançais, E., Hudrisier, D., Neyrolles, O. et al. Finding and filling the knowledge gaps in mechanisms of T cell-mediated TB immunity to inform vaccine design. Nat Rev Immunol 25, 798–815 (2025). https://doi.org/10.1038/s41577-025-01192-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-025-01192-z