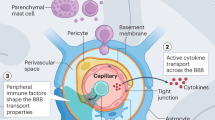
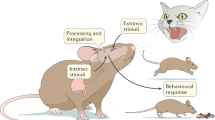
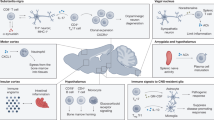
Abstract
Connections between the nervous and immune systems are increasingly recognized as central to brain–body physiology. In this Review, we examine how these systems collaborate to detect and respond to both internal and external stimuli — such as psychological stress, circadian cues, infection, and tissue injury. Rather than operating in isolation, the nervous and immune systems form an integrated network that is more than the sum of its parts. They share a common architecture and vocabulary, enabling bidirectional connection and communication that modulate immune cell characteristics throughout the body. We review immune–nervous interactions within two complementary frameworks: first, a spatial framework that distinguishes communication in the brain, communication within peripheral organs, and communication across distance; and second, a temporal framework that maps nervous system influence across the operational lifespan of the immune system — specifically focusing on how the nervous system impacts immune cell development, distribution, and execution of functions. Finally, we highlight key tools, clinical applications, and questions for future research on how both systems coordinate to respond to somatic and environmental stressors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dhabhar, F. S. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol. Res. 58, 193–210 (2014).
Haykin, H. & Rolls, A. The neuroimmune response during stress: a physiological perspective. Immunity 54, 1933–1947 (2021).
Chan, K. L., Poller, W. C., Swirski, F. K. & Russo, S. J. Central regulation of stress-evoked peripheral immune responses. Nat. Rev. Neurosci. 24, 591–604 (2023).
Koren, T. & Rolls, A. Immunoception: defining brain-regulated immunity. Neuron 110, 3425–3428 (2022).
Tracey, K. J. The inflammatory reflex. Nature 420, 853–859 (2002). This paper defines the ‘inflammatory reflex’, a neural circuit by which the vagus nerve can tune down and modulate immune responses.
Dantzer, R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol. Rev. 98, 477–504 (2018).
Bateman, A., Singh, A., Kral, T. & Solomon, S. The immune–hypothalamic–pituitary–adrenal axis. Endocr. Rev. 10, 92–112 (1989).
Blalock, J. E. The immune system as the sixth sense. J. Intern. Med. 257, 126–138 (2005).
Blalock, J. E. & Smith, E. M. Conceptual development of the immune system as a sixth sense. Brain Behav. Immun. 21, 23–33 (2007).
Dantzer, R. & Kelley, K. W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 21, 153–160 (2007).
Godinho-Silva, C., Cardoso, F. & Veiga-Fernandes, H. Neuro-immune cell units: a new paradigm in physiology. Annu. Rev. Immunol. 37, 19–46 (2019).
Turnbull, A. V. & Rivier, C. L. Regulation of the hypothalamic–pituitary–adrenal axis by cytokines: actions and mechanisms of action. Physiol. Rev. 79, 1–71 (1999).
Allen, N. J. & Lyons, D. A. Glia as architects of central nervous system formation and function. Science 362, 181–185 (2018).
Buchanan, J., da Costa, N. M. & Cheadle, L. Emerging roles of oligodendrocyte precursor cells in neural circuit development and remodeling. Trends Neurosci. 46, 628–639 (2023).
Doron, A. et al. Hippocampal astrocytes encode reward location. Nature 609, 772–778 (2022).
Krishnamurty, A. T. & Turley, S. J. Lymph node stromal cells: cartographers of the immune system. Nat. Immunol. 21, 369–380 (2020).
Morales-Sanchez, A., Shissler, S. C., Cowan, J. E. & Bhandoola, A. Revelations in thymic epithelial cell biology and heterogeneity from single-cell RNA sequencing and lineage tracing methodologies. Methods Mol. Biol. 2580, 25–49 (2023).
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Behan, P. O. & Behan, W. M. Neuroimmunology. Practitioner 226, 2044–2052 (1982).
Payan, D. G., McGillis, J. P. & Goetzl, E. J. Neuroimmunology. Adv. Immunol. 39, 299–323 (1986).
Li, Q. & Barres, B. A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 18, 225–242 (2018).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Chu, C., Artis, D. & Chiu, I. M. Neuro-immune interactions in the tissues. Immunity 52, 464–474 (2020).
Izumi, M., Nakanishi, Y., Kang, S. & Kumanogoh, A. Peripheral and central regulation of neuro-immune crosstalk. Inflamm. Regen. 44, 41 (2024).
Schiller, M., Ben-Shaanan, T. L. & Rolls, A. Neuronal regulation of immunity: why, how and where? Nat. Rev. Immunol. 21, 20–36 (2021).
Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213–1225 (2015).
Roth, B. L. DREADDs for neuroscientists. Neuron 89, 683–694 (2016).
Baysoy, A., Bai, Z., Satija, R. & Fan, R. The technological landscape and applications of single-cell multi-omics. Nat. Rev. Mol. Cell Biol. 24, 695–713 (2023).
Du, H. et al. Tuning immunity through tissue mechanotransduction. Nat. Rev. Immunol. 23, 174–188 (2023).
Logan, R. W. & McClung, C. A. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 20, 49–65 (2019).
Hodes, G. E. et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl Acad. Sci. USA 111, 16136–16141 (2014).
Sial, O. K., Warren, B. L., Alcantara, L. F., Parise, E. M. & Bolanos-Guzman, C. A. Vicarious social defeat stress: bridging the gap between physical and emotional stress. J. Neurosci. Methods 258, 94–103 (2016).
Sim, J. H. et al. Immune cell-stromal circuitry in lupus photosensitivity. J. Immunol. 206, 302–309 (2021).
Brumm, H., Goymann, W., Deregnaucourt, S., Geberzahn, N. & Zollinger, S. A. Traffic noise disrupts vocal development and suppresses immune function. Sci. Adv. 7, eabe2405 (2021).
Zhang, A. et al. The immune system can hear noise. Front. Immunol. 11, 619189 (2020).
Flayer, C. H. et al. A gammadelta T cell–IL-3 axis controls allergic responses through sensory neurons. Nature 634, 440–446 (2024).
Oetjen, L. K. et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 171, 217–228.e13 (2017).
Wang, F. & Kim, B. S. Itch: a paradigm of neuroimmune crosstalk. Immunity 52, 753–766 (2020).
Wang, F. et al. A basophil-neuronal axis promotes itch. Cell 184, 422–440.e17 (2021).
Xu, Z. H. et al. Mechanisms of the PD-1/PD-L1 pathway in itch: from acute itch model establishment to the role in chronic itch in mouse. Eur. J. Pharmacol. 960, 176128 (2023).
Eberl, G. A new age for (mucosal) neuroimmunology. Mucosal Immunol. 15, 1052–1055 (2022).
Fiore, N. T., Debs, S. R., Hayes, J. P., Duffy, S. S. & Moalem-Taylor, G. Pain-resolving immune mechanisms in neuropathic pain. Nat. Rev. Neurol. 19, 199–220 (2023).
Poller, W. C. et al. Brain motor and fear circuits regulate leukocytes during acute stress. Nature 607, 578–584 (2022). This study demonstrates how stress and motor circuits can influence rapid leukocyte redistribution in response to acute stress, profoundly influencing disease outcome.
Hart, B. L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137 (1988).
Franco, R., Pacheco, R., Lluis, C., Ahern, G. P. & O’Connell, P. J. The emergence of neurotransmitters as immune modulators. Trends Immunol. 28, 400–407 (2007).
Hodo, T. W., de Aquino, M. T. P., Shimamoto, A. & Shanker, A. Critical neurotransmitters in the neuroimmune network. Front. Immunol. 11, 1869 (2020).
Amado, J. A. et al. Blood levels of cytokines in brain-dead patients: relationship with circulating hormones and acute-phase reactants. Metabolism 44, 812–816 (1995).
Schwarz, P. et al. Brain death-induced inflammatory activity is similar to sepsis-induced cytokine release. Cell Transpl. 27, 1417–1424 (2018).
Weiss, S. et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am. J. Transpl. 7, 1584–1593 (2007).
Weiss, M. G. et al. Activation of the innate immune system in brain-dead donors can be reduced by luminal intestinal preservation during organ procurement surgery — a porcine model. Transpl. Int. 37, 13569 (2024).
Zitur, L. J. et al. Brain death enhances activation of the innate immune system and leads to reduced renal metabolic gene expression. Transplantation 103, 1821–1833 (2019).
Watts, R. P., Thom, O. & Fraser, J. F. Inflammatory signalling associated with brain dead organ donation: from brain injury to brain stem death and posttransplant ischaemia reperfusion injury. J. Transpl. 2013, 521369 (2013).
D’Aragon, F. et al. Effect of corticosteroid administration on neurologically deceased organ donors and transplant recipients: a systematic review and meta-analysis. BMJ Open 7, e014436 (2017).
Kotsch, K. et al. Methylprednisolone therapy in deceased donors reduces inflammation in the donor liver and improves outcome after liver transplantation: a prospective randomized controlled trial. Ann. Surg. 248, 1042–1050 (2008).
Kuecuek, O. et al. Significant reduction of proinflammatory cytokines by treatment of the brain-dead donor. Transpl. Proc. 37, 387–388 (2005).
Sowers, K. L., Gayda-Chelder, C. A. & Galantino, M. L. Self-reported cognitive impairment in individuals with primary immunodeficiency disease. Brain Behav. Immun. Health 9, 100170 (2020).
Sun, C.-L. et al. Adverse psychological outcomes in long-term survivors of hematopoietic cell transplantation: a report from the bone marrow transplant survivor study (BMTSS). Blood 118, 4723–4731 (2011).
Titman, P. et al. Cognitive and behavioral abnormalities in children after hematopoietic stem cell transplantation for severe congenital immunodeficiencies. Blood 112, 3907–3913 (2008).
Balaguer-Rosello, A. et al. Noninfectious neurologic complications after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 25, 1818–1824 (2019).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Frederick, N. M., Tavares, G. A. & Louveau, A. Neuroimmune signaling at the brain borders. Immunol. Rev. 311, 9–25 (2022).
Menard, C. et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 20, 1752–1760 (2017).
Cathomas, F. et al. Circulating myeloid-derived MMP8 in stress susceptibility and depression. Nature 626, 1108–1115 (2024). This work identifies monocyte-sourced MMP8 as a fundamental cytokine driving chronic stress-related inflammation in the brain as well as depression-like behaviors.
McKim, D. B. et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol. Psychiatry 23, 1421–1431 (2018).
Wohleb, E. S. et al. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry 75, 970–981 (2014).
Wohleb, E. S., Powell, N. D., Godbout, J. P. & Sheridan, J. F. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 33, 13820–13833 (2013).
Riazi, K. et al. Microglial activation and TNFα production mediate altered CNS excitability following peripheral inflammation. Proc. Natl Acad. Sci. USA 105, 17151–17156 (2008).
Hein, A. M. et al. Sustained hippocampal IL-1β overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav. Immun. 24, 243–253 (2010).
Varvel, N. H. et al. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc. Natl Acad. Sci. USA 113, E5665–E5674 (2016).
Chung, E. N. et al. Psychedelic control of neuroimmune interactions governing fear. Nature 641, 1276–1286 (2025).
D’Mello, C., Le, T. & Swain, M. G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 29, 2089–2102 (2009).
Huynh, P. et al. Myocardial infarction augments sleep to limit cardiac inflammation and damage. Nature 635, 168–177 (2024). This paper demonstrates that myocardial infarction induces monocytes to invade the brain, release TNF and induce sleep via TNF-sensing glutamatergic neurons, which protects against inflammation and cardiac damage.
Thorp, E. B. et al. CCR2+ monocytes promote white matter injury and cognitive dysfunction after myocardial infarction. Brain Behav. Immun. 119, 818–835 (2024). This study, together with that by Huynh et al. (2024), shows that monocytes to invade the brain via the choroid plexus after myocardial infarction, which can lead to white matter injury and cognitive dysfunction.
Garre, J. M., Silva, H. M., Lafaille, J. J. & Yang, G. CX3CR1+ monocytes modulate learning and learning-dependent dendritic spine remodeling via TNF-α. Nat. Med. 23, 714–722 (2017). This work shows that under inflammatory conditions, CX3CR1+ monocytes can home to the brain, release TNF-α and influence dendritic spine remodeling and cognitive dysfunction.
Pinho-Ribeiro, F. A. et al. Bacteria hijack a meningeal neuroimmune axis to facilitate brain invasion. Nature 615, 472–481 (2023). This study describes a novel way of bacteria to promote brain invasion by tricking nociceptive neurons to suppress meningeal macrophage inflammation via CGRP.
Wu, B. et al. Meningeal neutrophil immune signaling influences behavioral adaptation following threat. Neuron 113, 260–276 (2024).
Ito, M. et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 565, 246–250 (2019).
Kipnis, J., Gadani, S. & Derecki, N. C. Pro-cognitive properties of T cells. Nat. Rev. Immunol. 12, 663–669 (2012).
Kipnis, J., Cohen, H., Cardon, M., Ziv, Y. & Schwartz, M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl Acad. Sci. USA 101, 8180–8185 (2004).
Walsh, J. T. et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J. Clin. Invest. 125, 699–714 (2015).
Xia, M. et al. Elevated IL-22 as a result of stress-induced gut leakage suppresses septal neuron activation to ameliorate anxiety-like behavior. Immunity 58, 218–231.e12 (2025).
Prescott, S. L. & Liberles, S. D. Internal senses of the vagus nerve. Neuron 110, 579–599 (2022).
Kressel, A. M. et al. Identification of a brainstem locus that inhibits tumor necrosis factor. Proc. Natl Acad. Sci. USA 117, 29803–29810 (2020).
Olofsson, P. S. et al. α7 nicotinic acetylcholine receptor (α7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol. Med. 18, 539–543 (2012).
Pavlov, V. A. & Tracey, K. J. The vagus nerve and the inflammatory reflex-linking immunity and metabolism. Nat. Rev. Endocrinol. 8, 743–754 (2012).
Wang, H. et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2003).
Liu, Z., Han, B., Li, P., Wang, Z. & Fan, Q. Activation of α7nAChR by nicotine reduced the Th17 response in CD4+ T lymphocytes. Immunol. Invest. 43, 667–674 (2014).
Zhou, L. et al. Acetylcholine regulates the development of experimental autoimmune encephalomyelitis via the CD4+ cells proliferation and differentiation. Int. J. Neurosci. 130, 788–803 (2020).
Teratani, T. et al. The liver–brain–gut neural arc maintains the Tregcell niche in the gut. Nature 585, 591–596 (2020).
Zhu, Y. et al. A chemogenetic screen reveals that Trpv1-expressing neurons control regulatory T cells in the gut. Science 385, eadk1679 (2024).
Matteoli, G. et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63, 938–948 (2014).
Fielding, C. et al. Cholinergic signals preserve haematopoietic stem cell quiescence during regenerative haematopoiesis. Nat. Commun. 13, 543 (2022).
Su, Y. et al. Brainstem Dbh+ neurons control allergen-induced airway hyperreactivity. Nature 631, 601–609 (2024). This works maps the entire vagal circuit leading to bronchoconstriction following allergen exposure, regulated by adrenergic neurons in the nucleus of the solitary tract.
Alkaslasi, M. R. et al. Single nucleus RNA-sequencing defines unexpected diversity of cholinergic neuron types in the adult mouse spinal cord. Nat. Commun. 12, 2471 (2021).
Veerakumar, A., Yung, A. R., Liu, Y. & Krasnow, M. A. Molecularly defined circuits for cardiovascular and cardiopulmonary control. Nature 606, 739–746 (2022).
Cox, M. A. et al. Choline acetyltransferase-expressing T cells are required to control chronic viral infection. Science 363, 639–644 (2019).
Devi, S. et al. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 54, 1219–1230.e7 (2021).
Mendez-Ferrer, S., Chow, A., Merad, M. & Frenette, P. S. Circadian rhythms influence hematopoietic stem cells. Curr. Opin. Hematol. 16, 235–242 (2009).
Frodermann, V. et al. Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat. Med. 25, 1761–1771 (2019).
Maryanovich, M. et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 24, 782–791 (2018).
Nevin, J. T., Moussa, M., Corwin, W. L., Mandoiu, I. I. & Srivastava, P. K. Sympathetic nervous tone limits the development of myeloid-derived suppressor cells. Sci. Immunol. 5, eaay9368 (2020).
Ho, Y.-H. et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell 25, 407–418.e6 (2019).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Sestan, M. et al. Neuronal–ILC2 interactions regulate pancreatic glucagon and glucose homeostasis. Science 387, eadi3624 (2025).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Stanley, S. et al. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc. Natl Acad. Sci. USA 107, 7024–7029 (2010).
Ziegler, K. A. et al. Immune-mediated denervation of the pineal gland underlies sleep disturbance in cardiac disease. Science 381, 285–290 (2023). This paper describes a mechanism by which immune cells infiltrate the SNS-associated superior cervical ganglia during choric cardiac disease; axons that project from the superior cervical ganglia to the pineal gland are damaged, resulting in denervation of the pineal gland and disrupted sleep.
Baral, P., Udit, S. & Chiu, I. M. Pain and immunity: implications for host defence. Nat. Rev. Immunol. 19, 433–447 (2019).
Filtjens, J. et al. Nociceptive sensory neurons promote CD8 T cell responses to HSV-1 infection. Nat. Commun. 12, 2936 (2021).
Trankner, D., Hahne, N., Sugino, K., Hoon, M. A. & Zuker, C. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proc. Natl Acad. Sci. USA 111, 11515–11520 (2014).
Cohen, J. A. et al. Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell 178, 919–932.e14 (2019).
Lu, Y. Z. et al. CGRP sensory neurons promote tissue healing via neutrophils and macrophages. Nature 628, 604–611 (2024). This study shows how CGRP+ sensory neurons drive tissue repair by recruiting neutrophils and macrophages.
Kulalert, W. et al. The neuroimmune CGRP–RAMP1 axis tunes cutaneous adaptive immunity to the microbiota. Proc. Natl Acad. Sci. USA 121, e2322574121 (2024).
Perner, C. et al. Substance P release by sensory neurons triggers dendritic cell migration and initiates the type-2 immune response to allergens. Immunity 53, 1063–1077.e7 (2020).
Kashem, S. W. et al. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 43, 515–526 (2015).
Riol-Blanco, L. et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510, 157–161 (2014).
Hoeffel, G. et al. Sensory neuron-derived TAFA4 promotes macrophage tissue repair functions. Nature 594, 94–99 (2021).
Lai, N. Y. et al. Gut-innervating nociceptor neurons regulate Peyer’s patch microfold cells and SFB levels to mediate salmonella host defense. Cell 180, 33–49.e22 (2020).
Yang, D. et al. Nociceptor neurons direct goblet cells via a CGRP–RAMP1 axis to drive mucus production and gut barrier protection. Cell 185, 4190–4205.e25 (2022).
Baral, P. et al. Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 24, 417–426 (2018).
Mathur, S. et al. Nociceptor neurons promote IgE class switch in B cells. JCI Insight 6, e148510 (2021).
Nagashima, H. et al. Neuropeptide CGRP limits group 2 innate lymphoid cell responses and constrains type 2 inflammation. Immunity 51, 682–695.e6 (2019).
Pinho-Ribeiro, F. A. et al. Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell 173, 1083–1097.e22 (2018).
Talbot, S. et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 87, 341–354 (2015).
Wallrapp, A. et al. Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity 51, 709–723.e6 (2019).
Chiu, I. M. et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57 (2013). This work shows that bacterial activation of sensory neurons modulates pain and immune responses.
Crosson, T. et al. FcepsilonR1-expressing nociceptors trigger allergic airway inflammation. J. Allergy Clin. Immunol. 147, 2330–2342 (2021).
Serhan, N. et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 20, 1435–1443 (2019).
Tamari, M. et al. Sensory neurons promote immune homeostasis in the lung. Cell 187, 44–61.e17 (2024).
Crosson, T. et al. Cytokines reprogram airway sensory neurons in asthma. Cell Rep. 43, 115045 (2024).
Huang, S. et al. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell 184, 441–459.e25 (2021).
Wu, M. et al. Innervation of nociceptor neurons in the spleen promotes germinal center responses and humoral immunity. Cell 187, 2935–2951.e19 (2024). This work highlights a previously unexplored splenic nociceptive neurons critically enhancing germinal centre responses and antibody affinity.
Hou, Y. et al. Neuropeptide signalling orchestrates T cell differentiation. Nature 635, 444–452 (2024).
Hanc, P. et al. Multimodal control of dendritic cell functions by nociceptors. Science 379, eabm5658 (2023). This work reports direct electrical coupling, CCL2 and CGRP signalling as three distinct ways by which nociceptors regulate dendritic cells.
Wulfing, C. & Gunther, H. S. Dendritic cells and macrophages neurally hard-wired in the lymph node. Sci. Rep. 5, 16866 (2015).
Hulsmans, M. et al. Macrophages facilitate electrical conduction in the heart. Cell 169, 510–522.e20 (2017). This study identifies cardiac macrophages as key players in maintaining electrical conduction stability in the heart.
Hulsmans, M. et al. Recruited macrophages elicit atrial fibrillation. Science 381, 231–239 (2023).
Andersson, U. & Tracey, K. J. Neural reflexes in inflammation and immunity. J. Exp. Med. 209, 1057–1068 (2012).
Jarret, A. et al. Enteric nervous system-derived IL-18 orchestrates mucosal barrier immunity. Cell 180, 50–63.e12 (2020).
Jin, H., Li, M., Jeong, E., Castro-Martinez, F. & Zuker, C. S. A body–brain circuit that regulates body inflammatory responses. Nature 630, 695–703 (2024). This study describes previously unexplored cell populations in the vagal complex that control the inflammatory reflex.
Tracey, K. J. et al. Metabolic effects of cachectin/tumor necrosis factor are modified by site of production. Cachectin/tumor necrosis factor-secreting tumor in skeletal muscle induces chronic cachexia, while implantation in brain induces predominantly acute anorexia. J. Clin. Invest. 86, 2014–2024 (1990).
Blomqvist, A. Prostaglandin E2production by brain endothelial cells and the generation of fever. DNA Cell Biol. 42, 107–112 (2023).
Conti, B., Tabarean, I., Andrei, C. & Bartfai, T. Cytokines and fever. Front. Biosci. 9, 1433–1449 (2004).
Li, S. et al. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2−/−, but not in cyclooxygenase-1−/− mice. Brain Res. 825, 86–94 (1999).
Blatteis, C. M. & Sehic, E. Cytokines and fever. Ann. N. Y. Acad. Sci. 840, 608–618 (1998).
Luan, H. H. et al. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell 178, 1231–1244.e11 (2019). This paper shows that an immune cell product (GDF15) of inflammation signals to the area postrema in the brain and promotes sympathetic nervous system tone during sepsis, promoting cardiac function and survival.
Mendez-Ferrer, S., Lucas, D., Battista, M. & Frenette, P. S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447 (2008). This paper underscores the importance of the sympathetic nervous system tone in regulating circadian stem cell egress out of the bone marrow.
Farzi, A., Reichmann, F. & Holzer, P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta Physiol. 213, 603–627 (2015).
Procaccini, C., Pucino, V., De Rosa, V., Marone, G. & Matarese, G. Neuro-endocrine networks controlling immune system in health and disease. Front. Immunol. 5, 143 (2014).
Couvineau, A. et al. Orexins as novel therapeutic targets in inflammatory and neurodegenerative diseases. Front. Endocrinol. 10, 709 (2019).
McAlpine, C. S. et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387 (2019). This work demonstrates how sleep modulates hypocretin production, controlling haematopoiesis and reducing cardiovascular inflammation.
Sulek, K. Nobel prize for Edward Calvin Kendall, Thaddeus Reichstein and Philip Showalter Hench in 1950 for their discoveries related to adrenal cortex hormones, their structure and biological activity. Wiad. Lek. 21, 1885–1887 (1968).
Buttgereit, F. & Scheffold, A. Rapid glucocorticoid effects on immune cells. Steroids 67, 529–534 (2002).
Coutinho, A. E. & Chapman, K. E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell Endocrinol. 335, 2–13 (2011).
Aguilera, G. & Liu, Y. The molecular physiology of CRH neurons. Front. Neuroendocrinol. 33, 67–84 (2012).
Berson, S. A. & Yalow, R. S. Radioimmunoassay of ACTH in plasma. J. Clin. Invest. 47, 2725–2751 (1968).
Sheng, J. A. et al. The hypothalamic–pituitary–adrenal axis: development, programming actions of hormones, and maternal-fetal interactions. Front. Behav. Neurosci. 14, 601939 (2020).
Janssen, H. et al. Monocytes re-enter the bone marrow during fasting and alter the host response to infection. Immunity 56, 783–796.e7 (2023).
Janssen, H., Koekkoek, L. L. & Swirski, F. K. Effects of lifestyle factors on leukocytes in cardiovascular health and disease. Nat. Rev. Cardiol. 21, 157–169 (2024).
Chrousos, G. P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5, 374–381 (2009).
Dimsdale, J. E. & Moss, J. Plasma catecholamines in stress and exercise. JAMA 243, 340–342 (1980).
Nicholls, A. J., Wen, S. W., Hall, P., Hickey, M. J. & Wong, C. H. Y. Activation of the sympathetic nervous system modulates neutrophil function. J. Leukoc. Biol. 103, 295–309 (2018).
Ao, T. et al. Local sympathetic neurons promote neutrophil egress from the bone marrow at the onset of acute inflammation. Int. Immunol. 32, 727–736 (2020).
Chen, S. et al. Sympathetic stimulation facilitates thrombopoiesis by promoting megakaryocyte adhesion, migration, and proplatelet formation. Blood 127, 1024–1035 (2016).
Fu, W., Meng, G., Yang, X., Yu, L. & Jiang, H. Bone marrow sympathetic activation regulates post-myocardial infarction megakaryocyte expansion but not platelet production. Biochem. Biophys. Res. Commun. 513, 99–104 (2019).
Butterworth, M., McClellan, B. & Allansmith, M. Influence of sex in immunoglobulin levels. Nature 214, 1224–1225 (1967).
Forsyth, K. S., Jiwrajka, N., Lovell, C. D., Toothacre, N. E. & Anguera, M. C. The conneXion between sex and immune responses. Nat. Rev. Immunol. 24, 487–502 (2024).
Borba, V. V., Zandman-Goddard, G. & Shoenfeld, Y. Prolactin and autoimmunity. Front. Immunol. 9, 73 (2018).
Ngo, S. T., Steyn, F. J. & McCombe, P. A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 35, 347–369 (2014).
Taneja, V. Sex hormones determine immune response. Front. Immunol. 9, 1931 (2018).
Wenzek, C. et al. The interplay of thyroid hormones and the immune system — where we stand and why we need to know about it. Eur. J. Endocrinol. 186, R65–R77 (2022).
Palin, K. et al. Interleukin-6 activates arginine vasopressin neurons in the supraoptic nucleus during immune challenge in rats. Am. J. Physiol. Endocrinol. Metab. 296, E1289–E1299 (2009).
Froemke, R. C. & Young, L. J. Oxytocin, neural plasticity, and social behavior. Annu. Rev. Neurosci. 44, 359–381 (2021).
Boyd, J. H., Holmes, C. L., Wang, Y., Roberts, H. & Walley, K. R. Vasopressin decreases sepsis-induced pulmonary inflammation through the V2R. Resuscitation 79, 325–331 (2008).
Jankowski, M. et al. Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Res. Cardiol. 105, 205–218 (2010).
Oliveira-Pelegrin, G. R., Saia, R. S., Carnio, E. C. & Rocha, M. J. Oxytocin affects nitric oxide and cytokine production by sepsis-sensitized macrophages. Neuroimmunomodulation 20, 65–71 (2013).
Mehdi, S. F. et al. Oxytocin and related peptide hormones: candidate anti-inflammatory therapy in early stages of sepsis. Front. Immunol. 13, 864007 (2022).
Poutahidis, T. et al. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS ONE 8, e78898 (2013).
Bai, H. et al. Mechanical control of innate immune responses against viral infection revealed in a human lung alveolus chip. Nat. Commun. 13, 1928 (2022).
Muller, P. A. et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014).
Pinho, S. & Frenette, P. S. Haematopoietic stem cell activity and interactions with the niche. Nat. Rev. Mol. Cell Biol. 20, 303–320 (2019).
Yuan, J. & Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 25, 379–395 (2024).
Luster, A. D., Alon, R. & von Andrian, U. H. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 6, 1182–1190 (2005).
Moreau, H. D., Piel, M., Voituriez, R. & Lennon-Dumenil, A. M. Integrating physical and molecular insights on immune cell migration. Trends Immunol. 39, 632–643 (2018).
Arandjelovic, S. & Ravichandran, K. S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 16, 907–917 (2015).
Chen, S. et al. Macrophages in immunoregulation and therapeutics. Signal. Transduct. Target. Ther. 8, 207 (2023).
Lam, N., Lee, Y. & Farber, D. L. A guide to adaptive immune memory. Nat. Rev. Immunol. 24, 810–829 (2024).
Nutt, S. L., Hodgkin, P. D., Tarlinton, D. M. & Corcoran, L. M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160–171 (2015).
Paludan, S. R., Pradeu, T., Masters, S. L. & Mogensen, T. H. Constitutive immune mechanisms: mediators of host defence and immune regulation. Nat. Rev. Immunol. 21, 137–150 (2021).
Vivier, E. & Malissen, B. Innate and adaptive immunity: specificities and signaling hierarchies revisited. Nat. Immunol. 6, 17–21 (2005).
Maryanovich, M., Takeishi, S. & Frenette, P. S. Neural regulation of bone and bone marrow. Cold Spring Harb. Perspect. Med. 8, 2971–2973 (2018).
Ryu, V. et al. An atlas of brain–bone sympathetic neural circuits in mice. eLife 13, e95727 (2024).
Garcia-Garcia, A. et al. Dual cholinergic signals regulate daily migration of hematopoietic stem cells and leukocytes. Blood 133, 224–236 (2019).
Katayama, Y. et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006).
Yamazaki, S. et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158 (2011).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
Gerhardt, T., Huynh, P. & McAlpine, C. S. Neuroimmune circuits in the plaque and bone marrow regulate atherosclerosis. Cardiovasc. Res. 120, 2395–2407 (2024).
Gao, X. et al. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 589, 591–596 (2021). This work highlights the role of nociceptive nerves in regulating haematopoietic stem cell egress from the bone marrow.
Zhang, X. et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581, 204–208 (2020). This study reveals that tonic activation of brain stress centers can enhance antibody affinity following immunization.
De Virgiliis, F., Oliva, V. M., Kizil, B. & Scheiermann, C. Control of lymph node activity by direct local innervation. Trends Neurosci. 45, 704–712 (2022).
Pender, M. P. & Rist, M. J. Apoptosis of inflammatory cells in immune control of the nervous system: role of glia. Glia 36, 137–144 (2001).
Yshii, L., Gebauer, C., Bernard-Valnet, R. & Liblau, R. Neurons and T cells: understanding this interaction for inflammatory neurological diseases. Eur. J. Immunol. 45, 2712–2720 (2015).
Flugel, A. et al. Neuronal FasL induces cell death of encephalitogenic T lymphocytes. Brain Pathol. 10, 353–364 (2000).
Wagner, J. U. G. et al. Aging impairs the neurovascular interface in the heart. Science 381, 897–906 (2023).
Simats, A., Sager, H. B. & Liesz, A. Heart–brain axis in health and disease: role of innate and adaptive immunity. Cardiovasc. Res. 120, 2325–2335 (2025).
Akinyemi, D. E., Chevre, R. & Soehnlein, O. Neuro-immune crosstalk in hematopoiesis, inflammation, and repair. Trends Immunol. 45, 597–608 (2024).
Scheiermann, C., Kunisaki, Y. & Frenette, P. S. Circadian control of the immune system. Nat. Rev. Immunol. 13, 190–198 (2013).
Scheiermann, C. et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 37, 290–301 (2012). This paper demonstrates how adrenergic nerves control circadian oscillatory leukocyte trafficking into lymph nodes.
Nguyen, K. D. et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science 341, 1483–1488 (2013).
Druzd, D. et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 46, 120–132 (2017).
Dhabhar, F. S., Malarkey, W. B., Neri, E. & McEwen, B. S. Stress-induced redistribution of immune cells — from barracks to boulevards to battlefields: a tale of three hormones–Curt Richter Award winner. Psychoneuroendocrinology 37, 1345–1368 (2012).
Shi, D.-D. et al. Stress-induced red nucleus attenuation induces anxiety-like behavior and lymph node CCL5 secretion. Nat. Commun. 14, 6923 (2023).
Collins, N. et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178, 1088–1101.e15 (2019).
Jordan, S. et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 178, 1102–1114.e17 (2019). This study links dietary intake to changes in the inflammatory monocyte pool and systemic immunity.
Cavalcanti de Albuquerque, J. P. et al. Brain sensing of metabolic state regulates circulating monocytes. Sci. Immunol. 10, eadr3226 (2025).
Mass, E., Nimmerjahn, F., Kierdorf, K. & Schlitzer, A. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat. Rev. Immunol. 23, 563–579 (2023).
Chakarov, S. et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 (2019). This paper illustrates how there are different subpopulations of tissue resident macrophages in tissues that are in close proximity to nerves, suggesting an important role for tissue nerve homeostasis.
Fonseca, R. C. et al. Vagus nerve regulates the phagocytic and secretory activity of resident macrophages in the liver. Brain Behav. Immun. 81, 444–454 (2019).
Hu, D. et al. Distribution of nerve fibers and nerve-immune cell association in mouse spleen revealed by immunofluorescent staining. Sci. Rep. 10, 9850 (2020).
Iwai, H. et al. Tissue-resident M2 macrophages directly contact primary sensory neurons in the sensory ganglia after nerve injury. J. Neuroinflammation 18, 227 (2021).
Kolter, J. et al. A subset of skin macrophages contributes to the surveillance and regeneration of local nerves. Immunity 50, 1482–1497.e7 (2019).
Xue, Y. et al. The mouse autonomic nervous system modulates inflammation and epithelial renewal after corneal abrasion through the activation of distinct local macrophages. Mucosal Immunol. 11, 1496–1511 (2018).
Rosas-Ballina, M. et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl Acad. Sci. USA 105, 11008–11013 (2008).
Mallesh, S. et al. Sympathetic denervation alters the inflammatory response of resident muscularis macrophages upon surgical trauma and ameliorates postoperative ileus in mice. Int. J. Mol. Sci. 22, 6872 (2021).
Russo, S. et al. Stress-activated brain–gut circuits disrupt intestinal barrier integrity and social behaviour. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-3459170/v1 (2023).
Mohanta, S. K. et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 605, 152–159 (2022). This paper describes how a brain–body circuit of sensory afferent TRPV neurons and sympathetic efferent neurons senses and modulates leukocyte distribution and function in the adventitia of large vessels during atherosclerosis.
Mohanta, S. K., Yin, C., Weber, C. & Habenicht, A. J. R. Neuroimmune cardiovascular interfaces in atherosclerosis. Front. Cell Dev. Biol. 11, 1117368 (2023).
Kim, J. & Padanilam, B. J. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 87, 350–358 (2015).
Richards, A. M., Floyd, D. C., Terenghi, G. & McGrouther, D. A. Cellular changes in denervated tissue during wound healing in a rat model. Br. J. Dermatol. 140, 1093–1099 (1999).
Xiao, L. et al. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ. Res. 117, 547–557 (2015).
Huerta, T. S. et al. Neural representation of cytokines by vagal sensory neurons. Nat. Commun. 16, 3840 (2025).
Florsheim, E. B. et al. Immune sensing of food allergens promotes avoidance behaviour. Nature 620, 643–650 (2023).
Perrotta, S. et al. A heart–brain–spleen axis controls cardiac remodeling to hypertensive stress. Immunity 58, 648–665.e7 (2025).
Ben-Shaanan, T. L. et al. Activation of the reward system boosts innate and adaptive immunity. Nat. Med. 22, 940–944 (2016).
Ben-Shaanan, T. L. et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat. Commun. 9, 2723 (2018).
Carnevale, D. et al. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity 41, 737–752 (2014).
Carnevale, D. et al. A cholinergic–sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat. Commun. 7, 13035 (2016).
Gabanyi, I. et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164, 378–391 (2016).
Merlo, S. A., Belluscio, M. A., Pedreira, M. E. & Merlo, E. Memory persistence: from fundamental mechanisms to translational opportunities. Transl. Psychiatry 14, 98 (2024).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Koren, T. et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 184, 5902–5915.e17 (2021). This paper suggests that the brain has the capacity to store immune challenges as memories and that this has an impact on disease.
Rolls, A. Immunoception: the insular cortex perspective. Cell Mol. Immunol. 20, 1270–1276 (2023).
Jacobson, A., Yang, D., Vella, M. & Chiu, I. M. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 14, 555–565 (2021).
Simats, A. et al. Innate immune memory after brain injury drives inflammatory cardiac dysfunction. Cell 187, 4637–4655.e26 (2024).
Sammons, M. et al. Brain-body physiology: local, reflex, and central communication. Cell 187, 5877–5890 (2024).
Rae-Grant, A. et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology 90, 777–788 (2018).
Peterson, D. et al. Clinical safety and feasibility of a novel implantable neuroimmune modulation device for the treatment of rheumatoid arthritis: initial results from the randomized, double-blind, sham-controlled RESET-RA study. Bioelectron. Med. 10, 8 (2024).
Samuel, C., Cornman, H., Kambala, A. & Kwatra, S. G. A review on the safety of using JAK inhibitors in dermatology: clinical and laboratory monitoring. Dermatol. Ther. 13, 729–749 (2023).
Schwartz, D. M. et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 16, 843–862 (2017).
Wheeler, M. A. & Quintana, F. J. The neuroimmune connectome in health and disease. Nature 638, 333–342 (2025).
Kioussis, D. & Pachnis, V. Immune and nervous systems: more than just a superficial similarity? Immunity 31, 705–710 (2009).
Toni, R. The neuroendocrine system: organization and homeostatic role. J. Endocrinol. Invest. 27, 35–47 (2004).
Chapman, C. R., Tuckett, R. P. & Song, C. W. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J. Pain 9, 122–145 (2008).
Byron, N. & Sakata, S. Fiber photometry-based investigation of brain function and dysfunction. Neurophotonics 11, S11502 (2024).
Zong, W. et al. Large-scale two-photon calcium imaging in freely moving mice. Cell 185, 1240–1256.e30 (2022).
Russell, D. M., McCormick, D., Taberner, A. J., Malpas, S. C. & Budgett, D. M. A high bandwidth fully implantable mouse telemetry system for chronic ECG measurement. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2011, 7666–7669 (2011).
Berul, C. I., Aronovitz, M. J., Wang, P. J. & Mendelsohn, M. E. In vivo cardiac electrophysiology studies in the mouse. Circulation 94, 2641–2648 (1996).
DeNardo, L. A. et al. Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat. Neurosci. 22, 460–469 (2019).
Guenthner, C. J., Miyamichi, K., Yang, H. H., Heller, H. C. & Luo, L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784 (2013).
Saleeba, C., Dempsey, B., Le, S., Goodchild, A. & McMullan, S. A student’s guide to neural circuit tracing. Front. Neurosci. 13, 897 (2019).
Engel, E. A., Card, J. P. & Enquist, L. W. Transneuronal circuit analysis with pseudorabies viruses. Curr. Protoc. 3, e841 (2023).
Pomeranz, L. E., Reynolds, A. E. & Hengartner, C. J. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 69, 462–500 (2005).
Wojaczynski, G. J., Engel, E. A., Steren, K. E., Enquist, L. W. & Patrick Card, J. The neuroinvasive profiles of H129 (herpes simplex virus type 1) recombinants with putative anterograde-only transneuronal spread properties. Brain Struct. Funct. 220, 1395–1420 (2015).
Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, eabf1230 (2021).
Nakandakari-Higa, S. et al. Universal recording of immune cell interactions in vivo. Nature 627, 399–406 (2024).
Ueda, H. R. et al. Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 21, 61–79 (2020).
Winer, M. M. et al. In vivo noninvasive microscopy of human leucocytes. Sci. Rep. 7, 13031 (2017).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Ben-Shaanan, T., Schiller, M. & Rolls, A. Studying brain-regulation of immunity with optogenetics and chemogenetics; a new experimental platform. Brain Behav. Immun. 65, 1–8 (2017).
Kalogriopoulos, N. A. et al. Synthetic GPCRs for programmable sensing and control of cell behaviour. Nature 637, 230–239 (2025).
Heumos, L. et al. Best practices for single-cell analysis across modalities. Nat. Rev. Genet. 24, 550–572 (2023).
Marx, V. Method of the year: spatially resolved transcriptomics. Nat. Methods 18, 9–14 (2021).
McKinnon, K. M. Flow cytometry: an overview. Curr. Protoc. Immunol. 120, 5.1.1–5.1.11 (2018).
Spitzer, M. H. & Nolan, G. P. Mass cytometry: single cells, many features. Cell 165, 780–791 (2016).
Tomura, M. et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc. Natl Acad. Sci. USA 105, 10871–10876 (2008).
Yan, Y. et al. Macrophages excite muscle spindles with glutamate to bolster locomotion. Nature 637, 698–707 (2024).
Granton, E. et al. Biofilm exopolysaccharides alter sensory-neuron-mediated sickness during lung infection. Cell 187, 1874–1888.e14 (2024).
Wang, A. et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 166, 1512–1525.e12 (2016).
Raison, C. L. & Miller, A. H. The evolutionary significance of depression in pathogen host defense (PATHOS-D). Mol. Psychiatry 18, 15–37 (2013).
Zhu, X. A. et al. A neuroimmune circuit mediates cancer cachexia-associated apathy. Science 388, eadm8857 (2025).
O’Sullivan, D. et al. Fever supports CD8+ effector T cell responses by promoting mitochondrial translation. Proc. Natl Acad. Sci. USA 118, e2023752118 (2021).
Eisenberger, N. I. et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry 68, 748–754 (2010).
Maier, S. F. & Watkins, L. R. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol. Rev. 105, 83–107 (1998).
Miller, A. H., Haroon, E., Raison, C. L. & Felger, J. C. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress. Anxiety 30, 297–306 (2013).
Miller, A. H. & Raison, C. L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 (2016).
Imeri, L. & Opp, M. R. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 10, 199–210 (2009).
Ilanges, A. et al. Brainstem ADCYAP1+ neurons control multiple aspects of sickness behaviour. Nature 609, 761–771 (2022). This work reveals the role of ADCYAP1+ neurons in orchestrating sickness behaviour during systemic inflammation.
Osterhout, J. A. et al. A preoptic neuronal population controls fever and appetite during sickness. Nature 606, 937–944 (2022). This study identifies the preoptic nucleus of the hypothalamus as a master regulator of fever and appetite during illness.
Bin, N. R. et al. An airway-to-brain sensory pathway mediates influenza-induced sickness. Nature 615, 660–667 (2023). This paper describes a neural population of sensory neurons in the upper airways controlling sickness behaviour related to viral infection.
Boyle, C. C. et al. Inflammation and dimensions of reward processing following exposure to the influenza vaccine. Psychoneuroendocrinology 102, 16–23 (2019).
Jolink, T. A. et al. Inflammatory reactivity to the influenza vaccine is associated with changes in automatic social behavior. Brain Behav. Immun. 99, 339–349 (2022).
Lasselin, J. Back to the future of psychoneuroimmunology: studying inflammation-induced sickness behavior. Brain Behav. Immun. Health 18, 100379 (2021).
Lindsay, E. K. Small “doses” of inflammation initiate social sickness behavior. Brain Behav. Immun. 102, 40–41 (2022).
Acknowledgements
A.L. is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) no. 559481340. F.K.S. is funded by NHBLI grants P01 HL131478 and 1P01 HL142494. The authors wish to thank all members of the Swirski Lab who provided valuable input during discussions about this review. The authors thank K. Joyes for editing this manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks A. Rolls, C. Scheiermann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Atrioventricular node
-
A collection of cardiomyocytes that electrically connects the heart’s atria and ventricles to coordinate heart beating.
- Blalock’s ‘sixth sense’
-
The concept that the immune system acts like a sensory organ — detecting pathogens and inflammation — and communicates this information to the nervous system via shared signalling molecules.
- Blood–brain barrier
-
The blood–brain barrier (BBB) comprises multiple protective layers including endothelial cells and astrocytes, which play critical roles in regulating permeability and trafficking of cells and molecule between the blood and central nervous system.
- Choline acetyltransferase
-
An enzyme responsible for the synthesis of the neurotransmitter acetylcholine.
- Emergency haematopoiesis
-
A complex state of haematopoiesis consisting of a general expansion of haematopoietic stem and progenitor cells and a shift towards the myeloid lineage common to many inflammatory states.
- Exaptatic adaptations
-
Evolutionary repurposing of existing biological traits for new functions, such as immune molecules co-opted from developmental or metabolic pathways to serve host defence roles.
- Glymphatic system
-
A newly defined brain clearance pathway that is thought to remove waste and deliver nutrients to the brain.
- Inflammatory reflex
-
A neural feedback circuit, mediated by the vagus nerve, that detects and downregulates inflammation.
- Neuroimmune cell units
-
Anatomically distinct micro domains where immune cells and neurons interact to regulate normal the local tissue environment.
- Nucleus accumbens
-
A portion of the ventral striatum that receives dopamine innervation to coordinate responses to rewarding and aversive stimuli.
- Nucleus ambiguus
-
One of the two motor nuclei of the vagus nerve, projecting to internal organs like the heart and the lung.
- Paraventricular nucleus of the hypothalamus
-
Hypothalamic region that integrates neuroendocrine, autonomic and behavioural responses to stress, infection and inflammation by regulating the HPA axis and sympathetic tone.
- Red nucleus
-
A structure in the rostral midbrain involved in motor coordination.
- Suprachiasmatic nucleus
-
(SCN). A small region of the brain in the hypothalamus that is responsible for coordinating circadian rhythms. It receives light inputs from the retina, as well as inputs from other brain regions.
- Thalamus
-
A brain region that serves as a relay station to integrate sensory/motor information between the cortex and brainstem.
- Trained immunity
-
The long-term functional reprogramming of innate immune cells and bone marrow progenitors, which is evoked by exogenous or endogenous insults. It leads to an altered innate immune cell response towards a second challenge after the cell has returned to a non-activated state.
- Vagus nerve
-
The tenth cranial nerve, one of the major components of the parasympathetic nervous system, which regulates organ functions in the head, thoracic and abdominal cavity, and mediates communication between the brain and immune system, including the inflammatory reflex.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leunig, A., Gianeselli, M., Russo, S.J. et al. Connection and communication between the nervous and immune systems. Nat Rev Immunol 25, 912–933 (2025). https://doi.org/10.1038/s41577-025-01199-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-025-01199-6