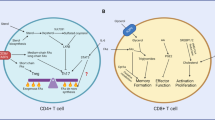
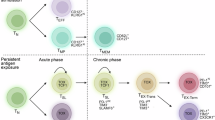
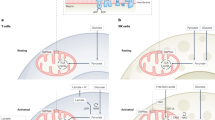
Abstract
Immunometabolism, the intersection of cellular metabolism and immune function, has revolutionized our understanding of T cell biology. Changes in cellular metabolism help guide the development of thymocytes and the transition of T cells from naive to effector, memory and tissue-resident states. Innate-like T cells are a unique group of T cells with special characteristics. They respond rapidly, reside mainly in tissues and express T cell receptors with limited diversity that recognize non-peptide antigens. This group includes invariant natural killer T (iNKT) cells, mucosal-associated invariant T (MAIT) cells and some populations of γδ T cells. Different subsets of innate-like T cells rely on specific metabolic pathways that influence their differentiation and function and distinguish them from conventional CD4+ and CD8+ T cells. Although there are differences between innate-like T cell types, they share metabolic and functional features. In this Review, we highlight recent research in this emerging field. Understanding how metabolic programmes differ between innate-like T cells and other T cells may open opportunities for tailoring innate-like T cell responses and adoptive T cell therapies for use in cancer, metabolic and autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Godfrey, D. I., Uldrich, A. P., McCluskey, J., Rossjohn, J. & Moody, D. B. The burgeoning family of unconventional T cells. Nat. Immunol. 16, 1114–1123 (2015).
LeBlanc, G., Kreissl, F. K., Melamed, J., Sobel, A. L. & Constantinides, M. G. The role of unconventional T cells in maintaining tissue homeostasis. Semin. Immunol. 61–64, 101656 (2022).
Hayday, A. C. & Vantourout, P. The innate biologies of adaptive antigen receptors. Annu. Rev. Immunol. 38, 487–510 (2020).
Gutierrez-Arcelus, M. et al. Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat. Commun. 10, 687 (2019).
Crosby, C. M. & Kronenberg, M. Tissue-specific functions of invariant natural killer T cells. Nat. Rev. Immunol. 18, 559–574 (2018).
Hayday, A. C. γδ T cell update: adaptate orchestrators of immune surveillance. J. Immunol. 203, 311–320 (2019).
Amini, A., Pang, D., Hackstein, C. P. & Klenerman, P. MAIT cells in barrier tissues: lessons from immediate neighbors. Front. Immunol. 11, 584521 (2020).
Nagarajan, N. A. & Kronenberg, M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J. Immunol. 178, 2706–2713 (2007).
Godfrey, D. I., Koay, H. F., McCluskey, J. & Gherardin, N. A. The biology and functional importance of MAIT cells. Nat. Immunol. 20, 1110–1128 (2019).
Loh, L. et al. Unraveling the phenotypic states of human innate-like T cells: comparative insights with conventional T cells and mouse models. Cell Rep. 43, 114705 (2024).
Lee, M. et al. Single-cell RNA sequencing identifies shared differentiation paths of mouse thymic innate T cells. Nat. Commun. 11, 4367 (2020).
Chandra, S. et al. Transcriptomes and metabolism define mouse and human MAIT cell populations. Sci. Immunol. 8, eabn8531 (2023). This work shows that at steady state, mouse MAIT1 and MAIT17 cells have distinct metabolic states, with MAIT17 cells having highly active mitochondria. Human MAIT cells are distinguished by lipid uptake and storage, but their metabolism is distinct to both mouse MAIT1 and MAIT17 cell subsets.
Garner, L. C. et al. Single-cell analysis of human MAIT cell transcriptional, functional and clonal diversity. Nat. Immunol. 24, 1565–1578 (2023).
Jayasinghe, R. G. et al. Single-cell transcriptomic profiling reveals diversity in human iNKT cells across hematologic tissues. Cell Rep. 44, 115587 (2025).
Kammann, T. et al. MAIT cell heterogeneity across paired human tissues reveals specialization of distinct regulatory and enhanced effector profiles. Sci. Immunol. 9, eadn2362 (2024).
Gray, J. I. et al. Human γδ T cells in diverse tissues exhibit site-specific maturation dynamics across the life span. Sci. Immunol. 9, eadn3954 (2024).
Stetson, D. B. et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198, 1069–1076 (2003).
Cheroutre, H., Lambolez, F. & Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11, 445–456 (2011).
Gras, S. et al. T cell receptor recognition of CD1b presenting a mycobacterial glycolipid. Nat. Commun. 7, 13257 (2016).
Lopez, K. et al. CD1b tetramers broadly detect T cells that correlate with mycobacterial exposure but not tuberculosis disease state. Front. Immunol. 11, 199 (2020).
Van Rhijn, I. et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat. Immunol. 14, 706–713 (2013).
Ascui, G. et al. Innateness transcriptome gradients characterize mouse T lymphocyte populations. J. Immunol. 214, 223–237 (2025).
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Bugaut, H. et al. A conserved transcriptional program for MAIT cells across mammalian evolution. J. Exp. Med. 221, e20231487 (2024).
LaMarche, N. M. et al. Distinct iNKT cell populations use IFNγ or ER stress-induced IL-10 to control adipose tissue homeostasis. Cell Metab. 32, 243–258.e246 (2020). This paper demonstrates that intracellular lipid accumulation in a subset of mouse iNKT cells in adipose tissue induces a stress response that drives IL-10, which is important for adipose tissue homeostasis.
Sag, D., Krause, P., Hedrick, C. C., Kronenberg, M. & Wingender, G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J. Clin. Invest. 124, 3725–3740 (2014).
Lynch, L. et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 37, 574–587 (2012).
Lynch, L. et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat. Immunol. 16, 85–95 (2015).
Murray, M. P. et al. Transcriptome and chromatin landscape of iNKT cells are shaped by subset differentiation and antigen exposure. Nat. Commun. 12, 1446 (2021).
Chang, P. P. et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 13, 35–43 (2011).
Rahimpour, A. et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 212, 1095–1108 (2015).
Carolan, E. et al. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J. Immunol. 194, 5775–5780 (2015).
Boulouis, C. et al. Human MAIT cell response profiles biased toward IL-17 or IL-10 are distinct effector states directed by the cytokine milieu. Proc. Natl Acad. Sci. USA 122, e2414230122 (2025).
Rhodes, K. A., Andrew, E. M., Newton, D. J., Tramonti, D. & Carding, S. R. A subset of IL-10-producing γδ T cells protect the liver from Listeria-elicited, CD8+ T cell-mediated injury. Eur. J. Immunol. 38, 2274–2283 (2008).
Kronenberg, M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu. Rev. Immunol. 23, 877–900 (2005).
Bendelac, A., Savage, P. B. & Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 (2007).
Brossay, L. et al. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188, 1521–1528 (1998).
Ferhat, M. H. et al. The impact of invariant NKT cells in sterile inflammation: the possible contribution of the alarmin/cytokine IL-33. Front. Immunol. 9, 2308 (2018).
Engel, I. et al. Innate-like functions of natural killer T cell subsets result from highly divergent gene programs. Nat. Immunol. 17, 728–739 (2016).
Gapin, L. Development of invariant natural killer T cells. Curr. Opin. Immunol. 39, 68–74 (2016).
Ngai, H. et al. LEF1 drives a central memory program and supports antitumor activity of natural killer T cells. Cancer Immunol. Res. 11, 171–183 (2023).
Tian, G. et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J. Clin. Invest. 126, 2341–2355 (2016).
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003).
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012).
Gold, M. C. et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 8, e1000407 (2010).
Le Bourhis, L. et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11, 701–708 (2010).
Corbett, A. J. et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509, 361–365 (2014).
Ito, E. et al. Sulfated bile acid is a host-derived ligand for MAIT cells. Sci. Immunol. 9, eade6924 (2024).
Constantinides, M. G. et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 366, eaax6624 (2019).
Cui, Y. et al. Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation. J. Clin. Invest. 125, 4171–4185 (2015).
Loh, L. et al. MAIT cells exacerbate chronic inflammation in a genetically diverse murine model of spontaneous colitis. Mucosal Immunol. 18, 958–972 (2025).
Provine, N. M. & Klenerman, P. MAIT cells in health and disease. Annu. Rev. Immunol. 38, 203–228 (2020).
Leng, T. et al. TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep. 28, 3077–3091.e5 (2019).
Lamichhane, R. et al. TCR- or cytokine-activated CD8+ mucosal-associated invariant T cells are rapid polyfunctional effectors that can coordinate immune responses. Cell Rep. 28, 3061–3076.e5 (2019).
Hinks, T. S. C. et al. Activation and in vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell Rep. 28, 3249–3262.e5 (2019).
Zhang, Y. et al. Mucosal-associated invariant T cells restrict reactive oxidative damage and preserve meningeal barrier integrity and cognitive function. Nat. Immunol. 23, 1714–1725 (2022).
Lu, B. et al. IL-17 production by tissue-resident MAIT cells is locally induced in children with pneumonia. Mucosal Immunol. 13, 824–835 (2020).
Yamana, S. et al. Mucosal-associated invariant T cells have therapeutic potential against ocular autoimmunity. Mucosal Immunol. 15, 351–361 (2022).
Gibbs, A. et al. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 10, 35–45 (2017).
Kelly, J. et al. Chronically stimulated human MAIT cells are unexpectedly potent IL-13 producers. Immunol. Cell Biol. 97, 689–699 (2019).
Chien, Y. H., Meyer, C. & Bonneville, M. γδ T cells: first line of defense and beyond. Annu. Rev. Immunol. 32, 121–155 (2014).
Hu, Y. et al. γδ T cells: origin and fate, subsets, diseases and immunotherapy. Signal. Transduct. Target. Ther. 8, 434 (2023).
Qu, G. et al. Comparing mouse and human tissue-resident γδ T cells. Front. Immunol. 13, 891687 (2022).
Prinz, I., Silva-Santos, B. & Pennington, D. J. Functional development of γδ T cells. Eur. J. Immunol. 43, 1988–1994 (2013).
Davey, M. S., Willcox, C. R., Baker, A. T., Hunter, S. & Willcox, B. E. Recasting human Vδ1 lymphocytes in an adaptive role. Trends Immunol. 39, 446–459 (2018).
Puan, K. J. et al. Preferential recognition of a microbial metabolite by human Vγ2Vδ2 T cells. Int. Immunol. 19, 657–673 (2007).
Di Marco Barros, R. et al. Epithelia use butyrophilin-like molecules to shape organ-specific γδ T cell compartments. Cell 167, 203–218.e17 (2016).
Vantourout, P. et al. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology. Proc. Natl Acad. Sci. USA 115, 1039–1044 (2018).
Harly, C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 120, 2269–2279 (2012).
Rigau, M., Uldrich, A. P. & Behren, A. Targeting butyrophilins for cancer immunotherapy. Trends Immunol. 42, 670–680 (2021).
Yuan, L. et al. Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells. Nature 621, 840–848 (2023).
Karunakaran, M. M. et al. A distinct topology of BTN3A IgV and B30.2 domains controlled by juxtamembrane regions favors optimal human γδ T cell phosphoantigen sensing. Nat. Commun. 14, 7617 (2023).
Makowski, L., Chaib, M. & Rathmell, J. C. Immunometabolism: from basic mechanisms to translation. Immunol. Rev. 295, 5–14 (2020).
O’Sullivan, D. The metabolic spectrum of memory T cells. Immunol. Cell Biol. 97, 636–646 (2019).
Buck, M. D., Sowell, R. T., Kaech, S. M. & Pearce, E. L. Metabolic instruction of immunity. Cell 169, 570–586 (2017).
Jameson, S. C., Hogquist, K. A. & Bevan, M. J. Positive selection of thymocytes. Annu. Rev. Immunol. 13, 93–126 (1995).
von Boehmer, H. & Fehling, H. J. Structure and function of the pre-T cell receptor. Annu. Rev. Immunol. 15, 433–452 (1997).
Zhang, M. et al. Metabolic regulation of T cell development. Front. Immunol. 13, 946119 (2022).
Wilfahrt, D. & Delgoffe, G. M. Metabolic waypoints during T cell differentiation. Nat. Immunol. 25, 206–217 (2024).
Clarke, A. J. & Simon, A. K. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat. Rev. Immunol. 19, 170–183 (2019).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Riffelmacher, T. & Simon, A. K. Mechanistic roles of autophagy in hematopoietic differentiation. FEBS J. 284, 1008–1020 (2017).
Riffelmacher, T., Richter, F. C. & Simon, A. K. Autophagy dictates metabolism and differentiation of inflammatory immune cells. Autophagy 14, 199–206 (2018).
Wei, D. G. et al. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J. Exp. Med. 202, 239–248 (2005).
Seach, N. et al. Double-positive thymocytes select mucosal-associated invariant T cells. J. Immunol. 191, 6002–6009 (2013).
Savage, A. K. et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 29, 391–403 (2008).
Oh-Hora, M. et al. Agonist-selected T cell development requires strong T cell receptor signaling and store-operated calcium entry. Immunity 38, 881–895 (2013).
Legoux, F. et al. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science 366, 494–499 (2019).
Veillette, A., Dong, Z. & Latour, S. Consequence of the SLAM–SAP signaling pathway in innate-like and conventional lymphocytes. Immunity 27, 698–710 (2007).
Nunez-Cruz, S. et al. Differential requirement for the SAP–Fyn interaction during NK T cell development and function. J. Immunol. 181, 2311–2320 (2008).
Koay, H. F. et al. A divergent transcriptional landscape underpins the development and functional branching of MAIT cells. Sci. Immunol. 4, eaay6039 (2019).
Pasquier, B. et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J. Exp. Med. 201, 695–701 (2005).
Nichols, K. E. et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat. Med. 11, 340–345 (2005).
Salou, M., Legoux, F. & Lantz, O. MAIT cell development in mice and humans. Mol. Immunol. 130, 31–36 (2021).
Constantinides, M. G. & Bendelac, A. Transcriptional regulation of the NKT cell lineage. Curr. Opin. Immunol. 25, 161–167 (2013).
Engel, I. & Kronenberg, M. Transcriptional control of the development and function of Vα14i NKT cells. Curr. Top. Microbiol. Immunol. 381, 51–81 (2014).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 168, 960–976 (2017).
Zhang, L. et al. Mammalian target of rapamycin complex 1 orchestrates invariant NKT cell differentiation and effector function. J. Immunol. 193, 1759–1765 (2014). This analysis of RAPTOR-deficient mice shows that mTORC1 is required for the differentiation of mouse iNKT cells but not of CD4+ and CD8+ thymocytes, which illustrates the unique metabolic requirements of iNKT cell differentiation.
Prevot, N. et al. Mammalian target of rapamycin complex 2 regulates invariant NKT cell development and function independent of promyelocytic leukemia zinc-finger. J. Immunol. 194, 223–230 (2015).
Wei, J., Yang, K. & Chi, H. Cutting edge: discrete functions of mTOR signaling in invariant NKT cell development and NKT17 fate decision. J. Immunol. 193, 4297–4301 (2014). This analysis of RICTOR-deficient mice shows the role of mTORC2 in iNKT cell differentiation, with a selective depletion of iNKT17 cells, which depend on PTEN regulation of mTORC2.
Park, H., Tsang, M., Iritani, B. M. & Bevan, M. J. Metabolic regulator Fnip1 is crucial for iNKT lymphocyte development. Proc. Natl Acad. Sci. USA 111, 7066–7071 (2014).
Salio, M. et al. Essential role for autophagy during invariant NKT cell development. Proc. Natl Acad. Sci. USA 111, E5678–E5687 (2014).
Pei, B. et al. Invariant NKT cells require autophagy to coordinate proliferation and survival signals during differentiation. J. Immunol. 194, 5872–5884 (2015).
Puleston, D. J. et al. Autophagy is a critical regulator of memory CD8+ T cell formation. eLife 3, e03706 (2014).
O’Sullivan, D. et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 (2014).
Raynor, J. L. et al. Hippo/Mst signaling coordinates cellular quiescence with terminal maturation in iNKT cell development and fate decisions. J. Exp. Med. 217, e20191157 (2020).
Weng, X. et al. Mitochondrial metabolism is essential for invariant natural killer T cell development and function. Proc. Natl Acad. Sci. USA 118, e2021385118 (2021).
Kanno, T. et al. ACC1-mediated fatty acid biosynthesis intrinsically controls thymic iNKT cell development. Int. Immunol. 36, 129–139 (2024).
Koh, J. et al. De novo fatty-acid synthesis protects invariant NKT cells from cell death, thereby promoting their homeostasis and pathogenic roles in airway hyperresponsiveness. eLife 12, RP87536 (2023).
Zhao, M. et al. Calcium signals regulate the functional differentiation of thymic iNKT cells. EMBO J. 40, e107901 (2021).
Pyaram, K. et al. Keap1–Nrf2 system plays an important role in invariant natural killer T cell development and homeostasis. Cell Rep. 27, 699–707 e694 (2019).
Sok, S. P. M. et al. Gpx4 regulates invariant NKT cell homeostasis and function by preventing lipid peroxidation and ferroptosis. J. Immunol. 213, 941–951 (2024).
Koay, H. F., Godfrey, D. I. & Pellicci, D. G. Development of mucosal-associated invariant T cells. Immunol. Cell Biol. 96, 598–606 (2018).
Tao, H. et al. Differential controls of MAIT cell effector polarization by mTORC1/mTORC2 via integrating cytokine and costimulatory signals. Nat. Commun. 12, 2029 (2021). This study shows that, similar to other mouse innate-like T cells, differentiation of mouse MAIT1 and MAIT17 cell subsets differentially depends on mTORCs, with MAIT17 cells more dependent on mTORC2, integrating signals from cytokines such as IL-1β and IL-23 to drive mTORC2 activation.
Salou, M. et al. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J. Exp. Med. 216, 133–151 (2019).
Harsha Krovi, S. et al. Thymic iNKT single cell analyses unmask the common developmental program of mouse innate T cells. Nat. Commun. 11, 6238 (2020).
Lee, Y. J. et al. Lineage-specific effector signatures of invariant NKT cells are shared amongst γδ T, innate lymphoid, and TH cells. J. Immunol. 197, 1460–1470 (2016).
Lopes, N. et al. Distinct metabolic programs established in the thymus control effector functions of γδ T cell subsets in tumor microenvironments. Nat. Immunol. 22, 179–192 (2021). Mouse γδ T cell subsets have different metabolic features that develop in the thymus; γδT1 cells depend on glycolysis and stronger TCR signals, whereas γδT17 cells have increased mitochondrial mass and activity. γδT17 cell numbers are increased in obese mice and promote tumour growth.
Munoz-Ruiz, M. et al. TCR signal strength controls thymic differentiation of discrete proinflammatory gammadelta T cell subsets. Nat. Immunol. 17, 721–727 (2016).
Yang, Q. et al. Roles of mTORC1 and mTORC2 in controlling gammadelta T1 and gammadelta T17 differentiation and function. Cell Death Differ. 27, 2248–2262 (2020). This research shows that both mouse γδT1 and γδT17 cells require mTORC1 for differentiation, but analysis of RICTOR-deficient mice indicates that mTORC2 deficiency selectively inhibits the generation of γδT17 cells.
Xiao, Z. et al. Lkb1 orchestrates γδ T-cell metabolic and functional fitness to control IL-17-mediated autoimmune hepatitis. Cell Mol. Immunol. 21, 546–560 (2024).
Yang, K. et al. Metabolic signaling directs the reciprocal lineage decisions of αβ and γδ T cells. Sci. Immunol. 3, eaas9818 (2018).
Blanco, D. B. et al. PTEN directs developmental and metabolic signaling for innate-like T cell fate and tissue homeostasis. Nat. Cell Biol. 24, 1642–1654 (2022).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Peng, M. et al. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354, 481–484 (2016).
Siska, P. J. & Rathmell, J. C. Metabolic signaling drives IFN-γ. Cell Metab. 24, 651–652 (2016).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Pan, Y. et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 252–256 (2017).
Frizzell, H. et al. Organ-specific isoform selection of fatty acid-binding proteins in tissue-resident lymphocytes. Sci. Immunol. 5, eaay9283 (2020).
Reina-Campos, M. et al. Metabolic programs of T cell tissue residency empower tumour immunity. Nature 621, 179–187 (2023).
Salerno, F. et al. Translational repression of pre-formed cytokine-encoding mRNA prevents chronic activation of memory T cells. Nat. Immunol. 19, 828–837 (2018).
Meermeier, E. W. et al. Human lung-resident mucosal-associated invariant T cells are abundant, express antimicrobial proteins, and are cytokine responsive. Commun. Biol. 5, 942 (2022).
Riffelmacher, T. & Kronenberg, M. Metabolic triggers of invariant natural killer T-cell activation during sterile autoinflammatory disease. Crit. Rev. Immunol. 40, 367–378 (2020).
Kumar, A. et al. Enhanced oxidative phosphorylation in NKT cells is essential for their survival and function. Proc. Natl Acad. Sci. USA 116, 7439–7448 (2019). This work shows that mouse splenic iNKT cells have a unique mode of glucose metabolism following antigen activation. Rather than generating lactic acid, they preferentially use the anabolic pentose phosphate pathway for their survival and cytokine production.
Khurana, P. et al. Distinct bioenergetic features of human invariant natural killer T cells enable retained functions in nutrient-deprived states. Front. Immunol. 12, 700374 (2021).
Fu, S. et al. Immunometabolism regulates TCR recycling and iNKT cell functions. Sci. Signal. 12, eaau1788 (2019).
Xie, D., Zhu, S. & Bai, L. Lactic acid in tumor microenvironments causes dysfunction of NKT cells by interfering with mTOR signaling. Sci. China Life Sci. 59, 1290–1296 (2016).
Fu, S. et al. Impaired lipid biosynthesis hinders anti-tumor efficacy of intratumoral iNKT cells. Nat. Commun. 11, 438 (2020).
Riffelmacher, T. et al. Divergent metabolic programmes control two populations of MAIT cells that protect the lung. Nat. Cell Biol. 25, 877–891 (2023). This work shows that antigen-adapted or memory-like mouse MAIT17 cells have increased mitochondrial activity. Metabolism influences the number and function of MAIT1 and MAIT17 cell subsets, with MAIT1 cells more dependent on glycolysis and MAIT17 cells more dependent on OXPHOS.
Konjar, S. et al. Mitochondria maintain controlled activation state of epithelial-resident T lymphocytes. Sci. Immunol. 3, eaan2543 (2018).
Lamichhane, R. et al. Human liver-derived MAIT cells differ from blood MAIT cells in their metabolism and response to TCR-independent activation. Eur. J. Immunol. 51, 879–892 (2021).
Deschler, S. et al. Polyunsaturated fatty acid-induced metabolic exhaustion and ferroptosis impair the anti-tumour function of MAIT cells in MASLD. J. Hepatol. https://doi.org/10.1016/j.jhep.2025.06.006 (2025). This work shows that MAIT cells lose their antitumour function in metabolic dysfunction-associated steatotic liver disease owing to PUFA-driven lipid peroxide accumulation, which induces metabolic exhaustion and ferroptosis. This reveals a new immunometabolic axis that impairs MAIT cell-mediated cancer surveillance and offers a therapeutic target to enhance liver cancer immunotherapy.
Ryan, E. K. et al. Iron is critical for mucosal-associated invariant T cell metabolism and effector functions. J. Immunol. 212, 1706–1713 (2024).
O’Brien, A. et al. Obesity reduces mTORC1 activity in mucosal-associated invariant T cells, driving defective metabolic and functional responses. J. Immunol. 202, 3404–3411 (2019). This report shows that the activation of peripheral blood human MAIT cells depends on amino acid uptake via the transporter SLC7A5, leading to mTORC1 activation, enhanced glucose uptake and glycolysis. All of these responses are decreased in MAIT cells from individuals with obesity.
Zhou, C. Y. et al. Peripheral blood MR1 tetramer-positive mucosal-associated invariant T-cell function is modulated by mammalian target of rapamycin complex 1 in patients with active tuberculosis. Immunology 173, 497–510 (2024).
Zinser, M. E. et al. Human MAIT cells show metabolic quiescence with rapid glucose-dependent upregulation of granzyme B upon stimulation. Immunol. Cell Biol. 96, 666–674 (2018).
Cassidy, F. C. et al. Glycogen-fuelled metabolism supports rapid mucosal-associated invariant T cell responses. Proc. Natl Acad. Sci. USA 120, e2300566120 (2023). The very early cytokine responses and cytotoxic activity of peripheral blood human MAIT cells depend on their ability to synthesize and store glycogen, and to rapidly break it down after TCR stimulation, a feature they share with memory T cells.
Kedia-Mehta, N. et al. The proliferation of human mucosal-associated invariant T cells requires a MYC–SLC7A5–glycolysis metabolic axis. Sci. Signal. 16, eabo2709 (2023). This work shows that MAIT cell proliferation and function depend on MYC-regulated amino acid transport and glycolysis, which is relevant to maintain MAIT cell function in obesity.
Howson, L. J. et al. Mucosal-associated invariant T cell effector function is an intrinsic cell property that can be augmented by the metabolic cofactor α-ketoglutarate. J. Immunol. 206, 1425–1435 (2021).
Brien, A. O. et al. Targeting mitochondrial dysfunction in MAIT cells limits IL-17 production in obesity. Cell Mol. Immunol. 17, 1193–1195 (2020).
Douguet, L. et al. Nitric oxide synthase 2 improves proliferation and glycolysis of peripheral γδ T cells. PLoS One 11, e0165639 (2016).
Laird, R. M., Wolf, B. J., Princiotta, M. F. & Hayes, S. M. γδ T cells acquire effector fates in the thymus and differentiate into cytokine-producing effectors in a Listeria model of infection independently of CD28 costimulation. PLoS One 8, e63178 (2013).
Kao, Y. S. et al. Metabolic reprogramming of interleukin-17-producing γδ T cells promotes ACC1-mediated de novo lipogenesis under psoriatic conditions. Nat. Metab. 7, 966–984 (2025). Psoriatic inflammation drives γδT17 cells to adopt aerobic glycolysis and fatty acid synthesis via ACC1, which mediates IL-17A production and inflammation.
Ibusuki, A., Kawai, K., Nitahara-Takeuchi, A., Arguello, R. J. & Kanekura, T. TCR signaling and cellular metabolism regulate the capacity of murine epidermal γδ T cells to rapidly produce IL-13 but not IFN-γ. Front. Immunol. 15, 1361139 (2024).
Aehnlich, P. et al. Glycolysis inhibition affects proliferation and cytotoxicity of Vγ9Vδ2 T cells expanded for adoptive cell therapy. Cytotherapy 26, 1033–1045 (2024).
Mu, X. et al. Glucose metabolism controls human γδ T-cell-mediated tumor immunosurveillance in diabetes. Cell Mol. Immunol. 19, 944–956 (2022). In Vγ9Vδ2 human γδ T cells, cytolytic capacity is inhibited by glycolysis generating excess lactate which inhibits AMPK. Excess lactic acid also inhibits mouse iNKT cells.
Shi, L. Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).
Ikejiri, A. et al. Dynamic regulation of TH17 differentiation by oxygen concentrations. Int. Immunol. 24, 137–146 (2012).
Chou, T. F. et al. Tumour suppressor death-associated protein kinase targets cytoplasmic HIF-1α for TH17 suppression. Nat. Commun. 7, 11904 (2016).
Xu, K. et al. Glycolytic ATP fuels phosphoinositide 3-kinase signaling to support effector T helper 17 cell responses. Immunity 54, 976–987.e7 (2021).
Xu, C. et al. Expansion of MAIT cells in the combined absence of NKT and γδ-T cells. Mucosal Immunol. 16, 446–461 (2023).
Lee, Y. J. et al. Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity 43, 566–578 (2015).
Clancy-Thompson, E. et al. Transnuclear mice reveal Peyer’s patch iNKT cells that regulate B-cell class switching to IgG1. EMBO J. 38, e101260 (2019).
Lebrusant-Fernandez, M. et al. IFN-γ-dependent regulation of intestinal epithelial homeostasis by NKT cells. Cell Rep. 43, 114948 (2024).
Nielsen, M. M., Witherden, D. A. & Havran, W. L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 17, 733–745 (2017).
Gao, M. & Zhao, X. Insights into the tissue repair features of MAIT cells. Front. Immunol. 15, 1432651 (2024).
Aguiar, C. F. et al. Tissue-specific metabolic profile drives iNKT cell function during obesity and liver injury. Cell Rep. 42, 112035 (2023). This work shows that adipose tissue iNKT cells have distinct metabolism compared with those in the spleen and liver. Adipose tissue iNKT cells depend on AMPK for IL-10 production, which is important for adipose tissue homeostasis.
Ko, J. S. et al. Palmitate inhibits arthritis by inducing t-bet and gata-3 mRNA degradation in iNKT cells via IRE1α-dependent decay. Sci. Rep. 7, 14940 (2017). This work shows that increased palmitate induces an endoplasmic reticulum stress response in iNKT cells that leads to the degradation of transcription factors that drive IFNγ and IL-4 synthesis, which ameliorates arthritis in a serum transfer mouse model.
Newton, R., Priyadharshini, B. & Turka, L. A. Immunometabolism of regulatory T cells. Nat. Immunol. 17, 618–625 (2016).
Cheng, X. et al. Long-chain acylcarnitines induce senescence of invariant natural killer T cells in hepatocellular carcinoma. Cancer Res. 83, 582–594 (2023).
Bruno, M. E. C. et al. Accumulation of γδ T cells in visceral fat with aging promotes chronic inflammation. Geroscience 44, 1761–1778 (2022).
Khairallah, C. et al. A blend of broadly-reactive and pathogen-selected Vγ4 Vδ1 T cell receptors confer broad bacterial reactivity of resident memory γδ T cells. Mucosal Immunol. 15, 176–187 (2022).
Comeau, K., Paradis, P. & Schiffrin, E. L. Human and murine memory γδ T cells: evidence for acquired immune memory in bacterial and viral infections and autoimmunity. Cell Immunol. 357, 104217 (2020).
Chen, Z. et al. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol. 10, 58–68 (2017).
King, I. L. et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat. Immunol. 13, 44–50 (2011).
Shimizu, K. et al. KLRG+ invariant natural killer T cells are long-lived effectors. Proc. Natl Acad. Sci. USA 111, 12474–12479 (2014).
Parekh, V. V. et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J. Clin. Invest. 115, 2572–2583 (2005).
Zhang, H. et al. Antigen priming induces functional reprogramming in iNKT cells via metabolic and epigenetic regulation: an insight into iNKT cell-based antitumor immunotherapy. Cancer Immunol. Res. 11, 1598–1610 (2023).
Kamii, Y. et al. IL-27 regulates the differentiation of follicular helper NKT cells via metabolic adaptation of mitochondria. Proc. Natl Acad. Sci. USA 121, e2313964121 (2024).
Zeng, H. & Chi, H. mTOR signaling in the differentiation and function of regulatory and effector T cells. Curr. Opin. Immunol. 46, 103–111 (2017).
DePeaux, K. & Delgoffe, G. M. Metabolic barriers to cancer immunotherapy. Nat. Rev. Immunol. 21, 785–797 (2021).
Lukasik, Z., Elewaut, D. & Venken, K. MAIT cells come to the rescue in cancer immunotherapy? Cancers 12, 413 (2020).
Ruf, B., Greten, T. F. & Korangy, F. Innate lymphoid cells and innate-like T cells in cancer—at the crossroads of innate and adaptive immunity. Nat. Rev. Cancer 23, 351–371 (2023).
Courtney, A. N., Tian, G. & Metelitsa, L. S. Natural killer T cells and other innate-like T lymphocytes as emerging platforms for allogeneic cancer cell therapy. Blood 141, 869–876 (2023).
Harmon, C. et al. γδ T cell dichotomy with opposing cytotoxic and wound healing functions in human solid tumors. Nat. Cancer 4, 1122–1137 (2023).
Corkish, C., Aguiar, C. F. & Finlay, D. K. Approaches to investigate tissue-resident innate lymphocytes metabolism at the single-cell level. Nat. Commun. 15, 10424 (2024).
Tupin, E., Kinjo, Y. & Kronenberg, M. The unique role of natural killer T cells in the response to microorganisms. Nat. Rev. Microbiol. 5, 405–417 (2007).
Davey, M. S. et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9– subsets. Nat. Commun. 9, 1760 (2018).
Acknowledgements
The authors thank the Marga and Walter Boll foundation and the Virnich foundation for supporting their research, and acknowledge grants from the US National Institutes of Health (P30 DK120515, R01 AI172112) and a grant from Kyowa Kirin, Inc. (KKNA-Kyowa Kirin North America).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to researching information for the article, writing and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Lucy Garner, Andrew Hogan, Paul Klenerman, Hui-Fern Koay and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Dendritic epidermal T cells
-
A population of unique, dendritic-shaped γδ T cells expressing an invariant Vγ5Vδ1 T cell receptor (TCR). They are selected in the thymus during fetal development in a SKINT1-dependent manner and migrate to the skin epidermis, where they become long-lived, self-renewing immune sentinels that respond to stressed or damaged keratinocytes.
- Fatty acid oxidation
-
(FAO; also known as β-oxidation). The catabolic process by which fatty acid molecules are broken down inside mitochondria to generate acetyl-coenzyme A (acetyl-CoA). Acetyl-CoA enters the tricarboxylic acid cycle (TCA cycle), generating NADH and FADH2, which are electron carriers used in the electron transport chain.
- Glycolysis
-
A set of metabolic reactions that occur in the cytoplasm and convert glucose to pyruvate or lactate.
- Innate-like T cells
-
(Also known as unconventional T cells). A special group of T cells that acquire effector functions in the thymus, provide rapid immune responses and are predominantly resident in tissues. They have invariant or semi-invariant T cell receptors (TCRs), and they recognize types of antigens that are different from the peptides presented by polymorphic MHC class I and class II proteins to conventional T cells.
- Invariant natural killer T cells
-
(iNKT cells). An innate-like T cell subset that is a specialized lineage of T cells with invariant T cell receptor (TCR) α-chains. They recognize lipid antigens presented by CD1d molecules and rapidly produce cytokines. They play crucial roles in immune responses against infections and other contexts.
- Mitochondrial membrane potential
-
An electric potential difference across the inner mitochondrial membrane that is crucial for ATP production. The potential drives protons back across the membrane through ATP synthase, enabling the phosphorylation of ADP to ATP. Changes in mitochondrial membrane potential are often indicators of mitochondrial activity, health and function, and can be used as a proxy for the mitochondrial energy status. A loss of mitochondrial membrane potential can indicate mitochondrial silencing, removal or dysfunction.
- mTOR
-
The catalytic subunit of two structurally distinct complexes: mTORC1 and mTORC2. mTORC1 includes mTOR, RAPTOR, mLST8, PRAS40 and DEPTOR; it regulates cell growth and metabolism; and is inhibited by rapamycin. mTORC2 is composed of mTOR, RICTOR, mLST8, mSIN1, PROTOR1, PROTOR2 and DEPTOR; it controls cell survival and cytoskeletal organization; and is unaffected by rapamycin.
- Mucosal-associated invariant T cells
-
(MAIT cells). An innate-like T cell subset characterized by their invariant T cell receptor (TCR) α-chain. They recognize vitamin B metabolites presented by MR1 protein. They may also recognize other antigens, such as sulfated cholesterol derivatives. They provide rapid responses that effect other cell types and are particularly frequent among human intrahepatic lymphocytes.
- Oxidative phosphorylation
-
(OXPHOS). The mitochondrial pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy to produce ATP. It depends on the reducing agents NADH and FADH2 that are generated during the tricarboxylic acid cycle (TCA cycle) to build a proton gradient across the inner mitochondrial membrane. This mitochondrial membrane potential fuels the electron transfer chain that is coupled to the synthesis of ATP through this electrochemical transmembrane gradient.
- Tricarboxylic acid cycle
-
(TCA cycle). A group of chemical reactions within mitochondria that help generate chemical energy in the form of ATP, which is provided by oxidizing the acetyl group of acetyl-coenzyme A (acetyl-CoA). The TCA cycle is involved in the metabolism of proteins, fats and carbohydrates.
- γδ T cells
-
A population of T cells characterized by expression of T cell receptors (TCRs) encoded by gene families with a limited repertoire of V genes. Not all γδ T cells are innate-like T cells; for example, human Vδ1+ γδ T cells or Vγ9–Vδ2+ γδ T cells display unexpected parallels with conventional αβ T cells, whereas, Vγ9+Vδ2+ γδ T cells are more innate-like. γδ T cells are found in tissues and the blood, and the prevalence of different Vγ and Vδ genes varies according to the cellular location. They recognize a broad range of antigens, but responses to processed peptides presented by polymorphic MHC molecules are not typical. Butyrophilins play a role in the activation of several γδ T cell subsets.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Riffelmacher, T., Kronenberg, M. Metabolic control of innate-like T cells. Nat Rev Immunol 26, 67–82 (2026). https://doi.org/10.1038/s41577-025-01219-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-025-01219-5