Abstract
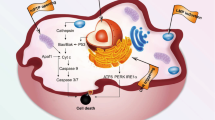
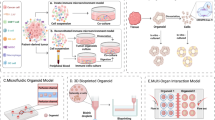
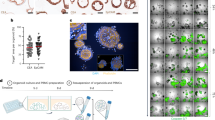
Organelles are the internal batteries, gears, actuators, 3D printers and transmitters that drive cell function. Their composition and activity vary between cell types depending on functional demands. In T cells, which are key mediators of immunosurveillance and tumour eradication, organelles are relatively few and function at basal levels when cells are at rest. However, upon activation, they increase in number and size and undergo extensive remodelling to support rapid proliferation, effector differentiation and adaptation to diverse microenvironments, including the tumour microenvironment, thereby enabling efficient clearance of target cells. In this Review, we provide an overview of recent advances in our understanding of how various organelles contribute to T cell-mediated antitumour immunity. We also discuss emerging strategies to modulate organelle functions — from organelle-targeted therapies and their use as cargo delivery systems to the transfer or transplantation of native or synthetic organelles — that have the potential to enhance cancer immunotherapies involving immune-checkpoint blockade or the adoptive transfer of T cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Suomalainen, A. & Nunnari, J. Mitochondria at the crossroads of health and disease. Cell 187, 2601–2627 (2024).
Steinert, E. M. et al. Mitochondrial respiration is necessary for CD8+ T cell proliferation and cell fate. Nat. Immunol. 26, 1267–1274 (2025).
Heuser, C., Renner, K., Kreutz, M. & Gattinoni, L. Targeting lactate metabolism for cancer immunotherapy — a matter of precision. Semin. Cancer Biol. 88, 32–45 (2023).
Simula, L. et al. Mitochondrial metabolism sustains CD8+ T cell migration for an efficient infiltration into solid tumors. Nat. Commun. 15, 2203 (2024).
Kaymak, I. et al. Carbon source availability drives nutrient utilization in CD8+ T cells. Cell Metab. 34, 1298–1311.e6 (2022).
Hunt, E. G. et al. Acetyl-CoA carboxylase obstructs CD8+ T cell lipid utilization in the tumor microenvironment. Cell Metab. 36, 969–983.e10 (2024).
Ma, S. et al. Targeting P4HA1 promotes CD8+ T cell progenitor expansion toward immune memory and systemic anti-tumor immunity. Cancer Cell 43, 213–231.e9 (2025).
Geiger, R. et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842.e13 (2016).
Kishton, R. J., Sukumar, M. & Restifo, N. P. Arginine arms T cells to thrive and survive. Cell Metab. 24, 647–648 (2016).
Martí i Líndez, A. A. et al. Mitochondrial arginase-2 is a cell-autonomous regulator of CD8+ T cell function and antitumor efficacy. JCI Insight 4, e132975 (2019).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Murphy, M. P. & Siegel, R. M. Mitochondrial ROS fire up T cell activation. Immunity 38, 201–202 (2013).
Scharping, N. E. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Yu, Y.-R. et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat. Immunol. 21, 1540–1551 (2020). Seminal studies by Scharping et al. (2021) and Yu et al. (2020) were instrumental in shaping our understanding of the role of mitochondrial dysfunction in driving T cell exhaustion.
Wu, H. et al. Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1α-mediated glycolytic reprogramming. Nat. Commun. 14, 6858 (2023).
Fisicaro, P. et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 23, 327–336 (2017).
Scharping, N. E. et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 45, 374–388 (2016).
Itoh, Y. et al. Mechanism of membrane-tethered mitochondrial protein synthesis. Science 371, 846–849 (2021).
Lisci, M. et al. Mitochondrial translation is required for sustained killing by cytotoxic T cells. Science 374, eabe9977 (2021).
Almeida, L. et al. Ribosome-targeting antibiotics impair T cell effector function and ameliorate autoimmunity by blocking mitochondrial protein synthesis. Immunity 54, 68–83.e6 (2021).
Martínez-Reyes, I. & Chandel, N. S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102 (2020).
Soriano-Baguet, L. & Brenner, D. Metabolism and epigenetics at the heart of T cell function. Trends Immunol. 44, 231–244 (2023).
Tyrakis, P. A. et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature 540, 236–241 (2016).
Minogue, E. et al. Glutarate regulates T cell metabolism and anti-tumour immunity. Nat. Metab. 5, 1747–1764 (2023).
Ryu, K. W. et al. Cellular ATP demand creates metabolically distinct subpopulations of mitochondria. Nature 635, 746–754 (2024). This study sheds light on how the machinery required for competing oxidative and reductive reactions in mitochondria is segregated into distinct mitochondrial populations under nutrient stress.
Katajisto, P. et al. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science 348, 340–343 (2015).
Hinge, A. et al. Asymmetrically segregated mitochondria provide cellular memory of hematopoietic stem cell replicative history and drive HSC attrition. Cell Stem Cell 26, 420–430.e6 (2020).
Borsa, M. et al. Inheritance of old mitochondria controls early CD8+ T cell fate commitment and is regulated by autophagy. Preprint at bioRxiv https://doi.org/10.1101/2024.01.29.577412 (2024).
Adams, W. C. et al. Anabolism-associated mitochondrial stasis driving lymphocyte differentiation over self-renewal. Cell Rep. 17, 3142–3152 (2016).
Xu, X. et al. Autophagy is essential for effector CD8+ T cell survival and memory formation. Nat. Immunol. 15, 1152–1161 (2014).
Schwarz, D. S. & Blower, M. D. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell. Mol. Life Sci. 73, 79–94 (2016).
Trebak, M. & Kinet, J. P. Calcium signalling in T cells. Nat. Rev. Immunol. 19, 154–169 (2019).
Ho, P. C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015).
Araki, K. et al. Translation is actively regulated during the differentiation of CD8+ effector T cells. Nat. Immunol. 18, 1046–1057 (2017).
Sinclair, L. V. & Cantrell, D. A. Protein synthesis and metabolism in T cells. Annu. Rev. Immunol. 43, 343–366 (2025).
Wolf, T. et al. Dynamics in protein translation sustaining T cell preparedness. Nat. Immunol. 21, 927–937 (2020).
Claiborne, M. D. et al. Persistent CAD activity in memory CD8+ T cells supports rRNA synthesis and ribosomal biogenesis required at rechallenge. Sci. Immunol. 7, eabh4271 (2022).
Todd, D. J., Lee, A.-H. & Glimcher, L. H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 8, 663–674 (2008).
Nair, K. A. & Liu, B. Navigating the landscape of the unfolded protein response in CD8+ T cells. Front. Immunol. 15, 1427859 (2024).
Correa-Medero, L. O. et al. ER-associated degradation adapter Sel1L is required for CD8+ T cell function and memory formation following acute viral infection. Cell Rep. 43, 114156 (2024).
Kamimura, D. & Bevan, M. J. Endoplasmic reticulum stress regulator XBP-1 contributes to effector CD8+ T cell differentiation during acute infection. J. Immunol. 181, 5433–5441 (2008).
Cao, Y. et al. ER stress-induced mediator C/EBP homologous protein thwarts effector T cell activity in tumors through T-bet repression. Nat. Commun. 10, 1280 (2019).
Fernández-Alfara, M. et al. Antitumor T-cell function requires CPEB4-mediated adaptation to chronic endoplasmic reticulum stress. EMBO J. 42, e111494 (2023). This study elucidates how T cells use CPEB4 to counteract physiological endoplasmic reticulum stress and maintain function by avoiding UPR-mediated dysfunction.
Mennerich, D., Kellokumpu, S. & Kietzmann, T. Hypoxia and reactive oxygen species as modulators of endoplasmic reticulum and Golgi homeostasis. Antioxid. Redox Signal. 30, 113–137 (2019).
Oberholtzer, N. et al. H2S-Prdx4 axis mitigates Golgi stress to bolster tumor-reactive T cell immunotherapeutic response. Sci. Adv. 10, eadp1152 (2024).
Wilson, C. et al. The Golgi apparatus: an organelle with multiple complex functions. Biochem. J. 433, 1–9 (2011).
Hong, Y. et al. ST3GAL1 and βII-spectrin pathways control CAR T cell migration to target tumors. Nat. Immunol. 24, 1007–1019 (2023).
Uslu, U. et al. The STING agonist IMSA101 enhances chimeric antigen receptor T cell function by inducing IL-18 secretion. Nat. Commun. 15, 3933 (2024).
Xu, N. et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J. Exp. Med. 218, e20200844 (2021).
Wang, X. et al. DNA sensing via the cGAS/STING pathway activates the immunoproteasome and adaptive T-cell immunity. Embo J. 42, e110597 (2023).
Richter, F., Paget, C. & Apetoh, L. STING-driven activation of T cells: relevance for the adoptive cell therapy of cancer. Cell Stress. 7, 95–104 (2023).
Li, W. et al. cGAS-STING-mediated DNA sensing maintains CD8+ T cell stemness and promotes antitumor T cell therapy. Sci. Transl. Med. 12, eaay9013 (2020).
Motedayen Aval, L., Pease, J. E., Sharma, R. & Pinato, D. J. Challenges and opportunities in the clinical development of STING agonists for cancer immunotherapy. J. Clin. Med. 9, 3323 (2020).
Fang, R. et al. Golgi apparatus-synthesized sulfated glycosaminoglycans mediate polymerization and activation of the cGAMP sensor STING. Immunity 54, 962–975.e8 (2021). The study identifies sulfated glycosaminoglycans as being essential for the translocation of STING from the endoplasmic reticulum to the Golgi apparatus and for modulating its activation.
Mindell, J. A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 74, 69–86 (2012).
Palmer, D. C. et al. Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance. J. Exp. Med. 212, 2095–2113 (2015).
Jin, J. et al. CISH impairs lysosomal function in activated T cells resulting in mitochondrial DNA release and inflammaging. Nat. Aging 3, 600–616 (2023).
Eskelinen, E.-L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Asp. Med. 27, 495–502 (2006).
Zhang, J. et al. Lysosomal LAMP proteins regulate lysosomal pH by direct inhibition of the TMEM175 channel. Mol. Cell 83, 2524–2539.e7 (2023).
Yu, J. et al. The OX40-TRAF6 axis promotes CTLA-4 degradation to augment antitumor CD8+ T-cell immunity. Cell. Mol. Immunol. 20, 1445–1456 (2023).
Isaaz, S., Baetz, K., Olsen, K., Podack, E. & Griffiths, G. M. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur. J. Immunol. 25, 1071–1079 (1995).
Chang, H.-F. et al. Identification of distinct cytotoxic granules as the origin of supramolecular attack particles in T lymphocytes. Nat. Commun. 13, 1029 (2022).
Sancak, Y. et al. Ragulator-rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 (2010).
Roczniak-Ferguson, A. et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 5, ra42 (2012).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Ham, H. et al. Lysosomal NKG7 restrains mTORC1 activity to promote CD8+ T cell durability and tumor control. Nat. Commun. 16, 1628 (2025).
Jin, J. et al. Activation of mTORC1 at late endosomes misdirects T cell fate decision in older individuals. Sci. Immunol. 6, eabg0791 (2021). This work uncovers how ageing reshapes mTORC1 compartmentalization, altering its association with lysosomes and endosomes to influence T cell differentiation.
Di Cara, F., Savary, S., Kovacs, W. J., Kim, P. & Rachubinski, R. A. The peroxisome: an up-and-coming organelle in immunometabolism. Trends Cell Biol. 33, 70–86 (2023).
Gilkrist, K. W., Opitz, J. M., Gilbert, E. F., Tsang, W. & Miller, P. Letter: immunodeficiency in the cerebro-hepato-renal syndrome of Zellweger. Lancet 1, 164–165 (1974).
Muri, J., Corak, B., Matsushita, M., Baes, M. & Kopf, M. Peroxisomes are critical for the development and maintenance of B1 and marginal zone B cells but dispensable for follicular B cells and T cells. J. Immunol. 208, 839–850 (2022).
Vicente-Manzanares, M. & Sánchez-Madrid, F. Role of the cytoskeleton during leukocyte responses. Nat. Rev. Immunol. 4, 110–122 (2004).
Martín-Cófreces, N. B., Baixauli, F. & Sánchez-Madrid, F. Immune synapse: conductor of orchestrated organelle movement. Trends Cell Biol. 24, 61–72 (2014).
Quintana, A. et al. T cell activation requires mitochondrial translocation to the immunological synapse. Proc. Natl Acad. Sci. Usa. 104, 14418–14423 (2007).
Butte, M. J., Stein, J. V. & Delon, J. The cytoskeleton in T cell migration and activation. Front. Immunol. 13, 1057533 (2022).
Wang, N., Butler, J. P. & Ingber, D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 (1993).
Shen, C. et al. A Dock8-dependent mechanosensitive central actin pool maintains T cell shape and protects the nucleus during migration. Sci. Immunol. 10, eadt9239 (2025).
Douanne, T. & Griffiths, G. M. Cytoskeletal control of the secretory immune synapse. Curr. Opin. Cell Biol. 71, 87–94 (2021).
Hammer, J. A., Wang, J. C., Saeed, M. & Pedrosa, A. T. Origin, organization, dynamics, and function of actin and actomyosin networks at the T cell immunological synapse. Annu. Rev. Immunol. 37, 201–224 (2019).
Thauland, T. J., Hu, K. H., Bruce, M. A. & Butte, M. J. Cytoskeletal adaptivity regulates T cell receptor signaling. Sci. Signal. 10, eaah3737 (2017).
Kumari, S. et al. Cytoskeletal tension actively sustains the migratory T-cell synaptic contact. EMBO J. 39, e102783 (2020).
Basu, R. et al. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell 165, 100–110 (2016).
Tello-Lafoz, M. et al. Cytotoxic lymphocytes target characteristic biophysical vulnerabilities in cancer. Immunity 54, 1037–1054.e7 (2021).
Hwang, S.-M. et al. Transgelin 2 guards T cell lipid metabolism and antitumour function. Nature 635, 1010–1018 (2024).
Combs, J. et al. Recruitment of dynein to the Jurkat immunological synapse. Proc. Natl Acad. Sci. USA 103, 14883–14888 (2006).
Hooikaas, P. J. et al. Kinesin-4 KIF21B limits microtubule growth to allow rapid centrosome polarization in T cells. eLife 9, e62876 (2020).
Scorrano, L. et al. Coming together to define membrane contact sites. Nat. Commun. 10, 1287 (2019).
Voeltz, G. K., Sawyer, E. M., Hajnóczky, G. & Prinz, W. A. Making the connection: how membrane contact sites have changed our view of organelle biology. Cell 187, 257–270 (2024).
Eisenberg-Bord, M., Shai, N., Schuldiner, M. & Bohnert, M. A tether is a tether is a tether: tethering at membrane contact sites. Dev. Cell 39, 395–409 (2016).
Wong, Y. C., Ysselstein, D. & Krainc, D. Mitochondria–lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 554, 382–386 (2018).
Hein, M. Y. et al. Global organelle profiling reveals subcellular localization and remodeling at proteome scale. Cell 188, 1137–1155.e20 (2025).
Booth, D. M., Várnai, P., Joseph, S. K. & Hajnóczky, G. Oxidative bursts of single mitochondria mediate retrograde signaling toward the ER. Mol. Cell 81, 3866–3876.e3862 (2021).
DiGiovanni, L. F. et al. ROS transfer at peroxisome-mitochondria contact regulates mitochondrial redox. Science 389, 157–162 (2025).
Wang, J. et al. Targeting membrane contact sites to mediate lipid dynamics: innovative cancer therapies. Cell Commun. Signal. 23, 89 (2025).
Martinvalet, D. The role of the mitochondria and the endoplasmic reticulum contact sites in the development of the immune responses. Cell Death Dis. 9, 336 (2018).
Bantug, G. R. et al. Mitochondria-endoplasmic reticulum contact sites function as immunometabolic hubs that orchestrate the rapid recall response of memory CD8+ T cells. Immunity 48, 542–555.e6 (2018).
Siddiqui, I. et al. Intratumoral Tcf1+PD-1+CD8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity 50, 195–211.e10 (2019).
Sade-Feldman, M. et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 175, 998–1013.e20 (2018).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Wang, Y. et al. Characteristics of premanufacture CD8+ T cells determine CAR-T efficacy in patients with diffuse large B-cell lymphoma. Signal. Transduct. Target. Ther. 8, 409 (2023).
Sukumar, M. et al. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 23, 63–76 (2016).
Si, X. et al. Mitochondrial isocitrate dehydrogenase impedes CAR T cell function by restraining antioxidant metabolism and histone acetylation. Cell Metab. 36, 176–192.e10 (2024). A mitochondria-focused compound screening that identifies IDH2 as an enasidenib-targetable checkpoint; IDH2 limits T cell memory by suppressing antioxidant metabolism and histone acetylation.
Jaccard, A. et al. Reductive carboxylation epigenetically instructs T cell differentiation. Nature 621, 849–856 (2023).
Wenes, M. et al. The mitochondrial pyruvate carrier regulates memory T-cell differentiation and antitumor function. Cell Metab. 34, 731–746.e9 (2022).
Wenes, M. et al. A novel mitochondrial pyruvate carrier inhibitor drives stem cell-like memory CAR T cell generation and enhances antitumor efficacy. Mol. Ther. Oncol. 32, 200897 (2024).
Hermans, D. et al. Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8+ T cell stemness and antitumor immunity. Proc. Natl Acad. Sci. USA 117, 6047–6055 (2020).
Frisch, A. T. et al. Redirecting glucose flux during in vitro expansion generates epigenetically and metabolically superior T cells for cancer immunotherapy. Cell Metab. 37, 870–885.e8 (2025).
Galluzzi, L., Guilbaud, E. & Garg, A. D. Mitochondrial succinate feeds T cell exhaustion in cancer. Cancer Cell 43, 168–170 (2025).
Funk, C. R. et al. PI3Kδ/γ inhibition promotes human CART cell epigenetic and metabolic reprogramming to enhance antitumor cytotoxicity. Blood 139, 523–537 (2022).
Pilipow, K. et al. Antioxidant metabolism regulates CD8+ T memory stem cell formation and antitumor immunity. JCI Insight 3, e122299 (2018).
Gollapudi, S. & Gupta, S. Reversal of oxidative stress-induced apoptosis in T and B lymphocytes by Coenzyme Q10 (CoQ10). Am. J. Clin. Exp. Immunol. 5, 41 (2016).
Finisguerra, V. et al. Metformin improves cancer immunotherapy by directly rescuing tumor-infiltrating CD8 T lymphocytes from hypoxia-induced immunosuppression. J. Immunother. Cancer 11, e005719 (2023).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Ma, J. et al. Lithium carbonate revitalizes tumor-reactive CD8+ T cells by shunting lactic acid into mitochondria. Nat. Immunol. 25, 552–561 (2024). This study provides deep mechanistic insights into how lithium carbonate modulates lysosomal function to counteract lactic acid-induced immunosuppression in the TME, making lactate available to CD8+T cells as an alternative energy source.
Dong, W. et al. D-mannose targets PD-1 to lysosomal degradation and enhances T cell-mediated anti-tumor immunity. Cancer Lett. 591, 216883 (2024).
Li, W. et al. Chimeric antigen receptor designed to prevent ubiquitination and downregulation showed durable antitumor efficacy. Immunity 53, 456–470.e6 (2020).
Wang, H. et al. Tonic ubiquitylation controls T-cell receptor: CD3 complex expression during T-cell development. EMBO J. 29, 1285–1298 (2010).
Katoh, Y. et al. Inhibition of stearoyl-CoA desaturase 1 (SCD1) enhances the antitumor T cell response through regulating β-catenin signaling in cancer cells and ER stress in T cells and synergizes with anti-PD-1 antibody. J. Immunother. Cancer 10, e004616 (2022). The study reveals a novel pharmacological approach to alleviate endoplasmic reticulum stress in T cells by inhibiting SCD1, thereby enhancing their antitumour efficacy.
Hurst, K. E. et al. Endoplasmic reticulum stress contributes to mitochondrial exhaustion of CD8+ T cells. Cancer Immunol. Res. 7, 476–486 (2019).
Tabdanov, E. D. et al. Engineering T cells to enhance 3D migration through structurally and mechanically complex tumor microenvironments. Nat. Commun. 12, 2815 (2021).
von Heijne, G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 5, 1335–1342 (1986).
Braulke, T. & Bonifacino, J. S. Sorting of lysosomal proteins. Biochim. Biophys. Acta Mol. Cell Res. 1793, 605–614 (2009).
Gould, S. J., Keller, G.-A., Hosken, N., Wilkinson, J. & Subramani, S. A conserved tripeptide sorts proteins to peroxisomes. J. Cell Biol. 108, 1657–1664 (1989).
Griffiths, G. et al. Localization of the Lys, Asp, Glu, Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J. Cell Biol. 127, 1557–1574 (1994).
Navarro, A. P. & Cheeseman, I. M. Identification of a Golgi-localized peptide reveals a minimal Golgi-targeting motif. Mol. Biol. Cell. 33, ar110 (2022).
Yang, J., Griffin, A., Qiang, Z. & Ren, J. Organelle-targeted therapies: a comprehensive review on system design for enabling precision oncology. Signal. Transduct. Target. Ther. 7, 379 (2022).
Kang, Y. C. et al. Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson’s disease models. Exp. Mol. Med. 50, 1–13 (2018).
Yu, H. et al. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber’s hereditary optic neuropathy in a mouse model. Proc. Natl Acad. Sci. USA 109, E1238–E1247 (2012).
Chuah, J.-A., Matsugami, A., Hayashi, F. & Numata, K. Self-assembled peptide-based system for mitochondrial-targeted gene delivery: functional and structural insights. Biomacromolecules 17, 3547–3557 (2016).
Fu, Y. et al. Engineering mtDNA deletions by reconstituting end joining in human mitochondria. Cell 188, 2778–2793.e21 (2025).
Escrig-Larena, J. I., Delgado-Pulido, S. & Mittelbrunn, M. Mitochondria during T cell aging. Semin. Immunol. 69, 101808 (2023).
Das, R. K., O’Connor, R. S., Grupp, S. A. & Barrett, D. M. Lingering effects of chemotherapy on mature T cells impair proliferation. Blood Adv. 4, 4653–4664 (2020).
Kam, W. W. & Banati, R. B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 65, 607–619 (2013).
Zhao, Q. et al. Target reprogramming lysosomes of CD8+ T cells by a mineralized metal-organic framework for cancer immunotherapy. Adv. Mater. 33, e2100616 (2021).
Sheikh, F. G., Pahan, K., Khan, M., Barbosa, E. & Singh, I. Abnormality in catalase import into peroxisomes leads to severe neurological disorder. Proc. Natl Acad. Sci. USA 95, 2961–2966 (1998).
Bhattacharyya, S. & Saha, J. Tumour, oxidative stress and host T cell response: cementing the dominance. Scand. J. Immunol. 82, 477–488 (2015).
Shah, R., Ibis, B., Kashyap, M. & Boussiotis, V. A. The role of ROS in tumor infiltrating immune cells and cancer immunotherapy. Metab. Clin. Exp. 151, 155747 (2024).
Shi, Y. et al. A vaccination with boosted cross presentation by ER-targeted antigen delivery for anti-tumor immunotherapy. Adv. Healthc. Mater. 10, e2001934 (2021).
Huang, Y. et al. The involvement of the mitochondrial membrane in drug delivery. Acta Biomater. 176, 28–50 (2024).
Malhotra, J. D. & Kaufman, R. J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 9, 2277–2294 (2007).
He, X., Li, J., An, S. & Jiang, C. pH-sensitive drug-delivery systems for tumor targeting. Ther. Deliv. 4, 1499–1510 (2013).
Siska, P. J. et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight 2, e93411 (2017).
Lee, J. & Song, C. H. Effect of reactive oxygen species on the endoplasmic reticulum and mitochondria during intracellular pathogen infection of mammalian cells. Antioxidants 10, 872 (2021).
Zhao, Y. et al. Pathogen infection-responsive nanoplatform targeting macrophage endoplasmic reticulum for treating life-threatening systemic infection. Nano Res. 15, 6243–6255 (2022).
Dumauthioz, N. et al. Enforced PGC-1α expression promotes CD8 T cell fitness, memory formation and antitumor immunity. Cell. Mol. Immunol. 18, 1761–1771 (2021).
Lontos, K. et al. Metabolic reprogramming via an engineered PGC-1α improves human chimeric antigen receptor T-cell therapy against solid tumors. J. Immunother. Cancer 11, e006522 (2023).
Pretto, S. et al. A functional single-cell metabolic survey identifies Elovl1 as a target to enhance CD8+ T cell fitness in solid tumours. Nat. Metab. 7, 508–530 (2025).
Huang, H. et al. In vivo CRISPR screening reveals nutrient signaling processes underpinning CD8+ T cell fate decisions. Cell 184, 1245–1261.e21 (2021).
Woodsworth, D. J., Dreolini, L., Abraham, L. & Holt, R. A. Targeted cell-to-cell delivery of protein payloads via the granzyme-perforin pathway. Mol. Ther. Methods Clin. Dev. 7, 132–145 (2017).
Huang, B. et al. Designed endocytosis-inducing proteins degrade targets and amplify signals. Nature 638, 796–804 (2025).
Piraner, D. I. et al. Engineered receptors for soluble cellular communication and disease sensing. Nature 638, 805–813 (2025).
Tavassolifar, M. J., Vodjgani, M., Salehi, Z. & Izad, M. The influence of reactive oxygen species in the immune system and pathogenesis of multiple sclerosis. Autoimmune Dis. 2020, 5793817 (2020).
Bai, R. & Cui, J. Mitochondrial immune regulation and anti-tumor immunotherapy strategies targeting mitochondria. Cancer Lett. 564, 216223 (2023).
Ali Hosseini Rad, S. M., Poudel, A., Tan, G. M. Y. & McLellan, A. D. Optimisation of Tet-On inducible systems for sleeping beauty-based chimeric antigen receptor (CAR) applications. Sci. Rep. 10, 13125 (2020).
Morciano, G. et al. Intersection of mitochondrial fission and fusion machinery with apoptotic pathways: role of Mcl-1. Biol. Cell 108, 279–293 (2016).
Kim, E. H., Neldner, B., Gui, J., Craig, R. W. & Suresh, M. Mcl-1 regulates effector and memory CD8 T-cell differentiation during acute viral infection. Virology 490, 75–82 (2016).
Tripathi, P., Koss, B., Opferman, J. T. & Hildeman, D. A. Mcl-1 antagonizes Bax/Bak to promote effector CD4+ and CD8+ T-cell responses. Cell Death Differ. 20, 998–1007 (2013).
Chatzispyrou, I. A., Held, N. M., Mouchiroud, L., Auwerx, J. & Houtkooper, R. H. Tetracycline antibiotics impair mitochondrial function and its experimental use confounds research. Cancer Res. 75, 4446–4449 (2015).
Moullan, N. et al. Tetracyclines disturb mitochondrial function across eukaryotic models: a call for caution in biomedical research. Cell Rep. 10, 1681–1691 (2015).
Ottina, E. et al. DNA-binding of the Tet-transactivator curtails antigen-induced lymphocyte activation in mice. Nat. Commun. 8, 1028 (2017).
Schmitt, A., Schulze-Osthoff, K. & Hailfinger, S. Correspondence: T cells are compromised in tetracycline transactivator transgenic mice. Cell Death Differ. 25, 634–636 (2018).
Li, H. S. et al. Multidimensional control of therapeutic human cell function with synthetic gene circuits. Science 378, 1227–1234 (2022).
Tang, L., Pan, S., Wei, X., Xu, X. & Wei, Q. Arming CAR-T cells with cytokines and more: innovations in the fourth-generation CAR-T development. Mol. Ther. 31, 3146–3162 (2023).
Greenshpan, Y. et al. Synthetic promoters to induce immune-effectors into the tumor microenvironment. Commun. Biol. 4, 143 (2021).
He, H. et al. Conditioned CAR-T cells by hypoxia-inducible transcription amplification (HiTA) system significantly enhances systemic safety and retains antitumor efficacy. J. Immunother. Cancer 9, e002755 (2021).
Bacman, S. R., Williams, S. L., Pinto, M., Peralta, S. & Moraes, C. T. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat. Med. 19, 1111–1113 (2013).
Gammage, P. A., Rorbach, J., Vincent, A. I., Rebar, E. J. & Minczuk, M. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol. Med. 6, 458–466 (2014).
Mok, B. Y. et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 583, 631–637 (2020).
Cho, S. I. et al. Targeted A-to-G base editing in human mitochondrial DNA with programmable deaminases. Cell 185, 1764–1776.e12 (2022).
Schmiderer, L., Yudovich, D., Oburoglu, L., Hjort, M. & Larsson, J. Site-specific CRISPR-based mitochondrial DNA manipulation is limited by gRNA import. Sci. Rep. 12, 18687 (2022).
Zhang, X. et al. Precise modelling of mitochondrial diseases using optimized mitoBEs. Nature 639, 735–745 (2025). mtDNA editing had previously been constrained to nuclease-based methods; the studies by Mok et al. (2020), Cho et al. (2022) and Zhang et al. (2025) demonstrate precise, non-destructive base editing as a breakthrough alternative.
Spees, J. L., Olson, S. D., Whitney, M. J. & Prockop, D. J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl Acad. Sci. USA 103, 1283–1288 (2006).
Borcherding, N. & Brestoff, J. R. The power and potential of mitochondria transfer. Nature 623, 283–291 (2023).
Berridge, M. V. et al. Horizontal mitochondrial transfer in cancer biology: potential clinical relevance. Cancer Cell 43, 803–807 (2025).
Nakai, R. et al. Mitochondria transfer-based therapies reduce the morbidity and mortality of Leigh syndrome. Nat. Metab. 6, 1886–1896 (2024).
Nicolás-Ávila, J. A. et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109.e23 (2020).
Hoover, G. et al. Nerve-to-cancer transfer of mitochondria during cancer metastasis. Nature 644, 252–262 (2025).
Saha, T. et al. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nat. Nanotechnol. 17, 98–106 (2022).
Zhang, H. et al. Systematic investigation of mitochondrial transfer between cancer cells and T cells at single-cell resolution. Cancer Cell 41, 1788–1802.e10 (2023).
Ikeda, H. et al. Immune evasion through mitochondrial transfer in the tumour microenvironment. Nature 638, 225–236 (2025).
Baldwin, J. G. et al. Intercellular nanotube-mediated mitochondrial transfer enhances T cell metabolic fitness and antitumor efficacy. Cell 187, 6614–6630.e21 (2024). Studies by Ikeda et al. (2025) and Baldwin et al. (2024) highlight the dynamic mitochondrial exchange between T cells and mesenchymal stromal cells or tumour cells, unveiling new therapeutic opportunities to enhance T cell fitness and antitumour immunity.
Court, A. C. et al. Survival advantage of native and engineered T cells is acquired by mitochondrial transfer from mesenchymal stem cells. J. Transl. Med. 22, 868 (2024).
Lin, R.-Z. et al. Mitochondrial transfer mediates endothelial cell engraftment through mitophagy. Nature 629, 660–668 (2024).
Durose, W. et al. First documentation of cell-to-cell peroxisome transfer in hematopoietic stem and progenitor cells: a method to relieve stress? Blood 140, 1686–1687 (2022).
Kumar, S. et al. Programmed exosome fusion for energy generation in living cells. Nat. Catal. 4, 763–774 (2021).
Wang, J. et al. Human neural stem cell-derived artificial organelles to improve oxidative phosphorylation. Nat. Commun. 15, 7855 (2024).
Oerlemans, R. A., Timmermans, S. B. & van Hest, J. C. Artificial organelles: towards adding or restoring intracellular activity. ChemBioChem 22, 2051–2078 (2021).
Simmel, F. C. Synthetic organelles. Emerg. Top. Life Sci. 3, 587–595 (2019).
Van Oppen, L. M. et al. Biodegradable synthetic organelles demonstrate ROS shielding in human-complex-I-deficient fibroblasts. ACS Cent. Sci. 4, 917–928 (2018).
Yang, S. et al. Construction of liposome-based extracellular artificial organelles on individual living cells. Angew. Chem. Int. Ed. Engl. 64, e202415823 (2025).
Sigmund, F. et al. Bacterial encapsulins as orthogonal compartments for mammalian cell engineering. Nat. Commun. 9, 1990 (2018). This study provides compelling proof-of-concept for a ‘bottom-up’ approach to genetically encode synthetic organelles that can self-assemble within the cell, enabling applications such as molecular imaging and beyond.
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Ogando, J. et al. PD-1 signaling affects cristae morphology and leads to mitochondrial dysfunction in human CD8+ T lymphocytes. J. Immunother. Cancer 7, 151 (2019).
Paillon, N. et al. PD-1 inhibits T cell actin remodeling at the immunological synapse independently of its signaling motifs. Sci. Signal. 16, eadh2456 (2023).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Loschinski, R. et al. IL-21 modulates memory and exhaustion phenotype of T-cells in a fatty acid oxidation-dependent manner. Oncotarget 9, 13125–13138 (2018).
Guo, Y. et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat. Immunol. 22, 746–756 (2021).
Monson, K. R. et al. Inherited mitochondrial genetics as a predictor of immune checkpoint inhibition efficacy in melanoma. Nat. Med. 31, 2385–2396 (2025).
Henning, A. N., Roychoudhuri, R. & Restifo, N. P. Epigenetic control of CD8+ T cell differentiation. Nat. Rev. Immunol. 18, 340–356 (2018).
Tavakoli, A., Hu, S., Ebrahim, S. & Kachar, B. Hemifusomes and interacting proteolipid nanodroplets mediate multi-vesicular body formation. Nat. Commun. 16, 4609 (2025).
Heinrich, L. et al. Whole-cell organelle segmentation in volume electron microscopy. Nature 599, 141–146 (2021). This study introduces deep learning models capable of registering 35 organelle classes in microscopy images across diverse resolutions and cell types, with open-source tools made publicly available for broader research use.
Lu, C. et al. Diffusion-based deep learning method for augmenting ultrastructural imaging and volume electron microscopy. Nat. Commun. 15, 4677 (2024).
Alon, S. et al. Expansion sequencing: spatially precise in situ transcriptomics in intact biological systems. Science 371, eaax2656 (2021).
Louvel, V. et al. iU-ExM: nanoscopy of organelles and tissues with iterative ultrastructure expansion microscopy. Nat. Commun. 14, 7893 (2023).
Lemaître, F. et al. Unveiling the molecular architecture of T cells and immune synapses with cryo-expansion microscopy. Preprint at bioRxiv https://doi.org/10.1101/2025.04.15.648816 (2025).
Büttner, M. et al. Challenges of using expansion microscopy for super-resolved imaging of cellular organelles. ChemBioChem 22, 686–693 (2021).
Zimmermann, J. A. et al. Functional multi-organelle units control inflammatory lipid metabolism of macrophages. Nat. Cell Biol. 26, 1261–1273 (2024).
Valm, A. M. et al. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 546, 162–167 (2017).
Lefebvre, A. E. Y. T. et al. Nellie: automated organelle segmentation, tracking and hierarchical feature extraction in 2D/3D live-cell microscopy. Nat. Methods 22, 751–763 (2025).
Garcia Romeu, H., Deville, S. & Salvati, A. Time- and space-resolved flow-cytometry of cell organelles to quantify nanoparticle uptake and intracellular trafficking by cells. Small 17, e2100887 (2021).
Schraivogel, D. et al. High-speed fluorescence image-enabled cell sorting. Science 375, 315–320 (2022). This work redefines live-cell sorting by introducing high-speed subcellular cytometry as a tool for profiling and selecting cells based on organelle morphometry.
Song, D. et al. Identification of an endoplasmic reticulum stress-related gene signature to evaluate the immune status and predict the prognosis of hepatocellular carcinoma. Front. Genet. 13, 850200 (2022).
Huang, Y. et al. Characterizing cancer metabolism from bulk and single-cell RNA-seq data using METAFlux. Nat. Commun. 14, 4883 (2023).
Jiang, A., Lyu, C. & Zhao, Y. Predicting T cell mitochondria hijacking from tumor single-cell RNA sequencing data with MitoR. Mathematics 13, 673 (2025).
Liu, W., He, H. & Chicco, D. Gene signatures for cancer research: a 25-year retrospective and future avenues. PLoS Comput. Biol. 20, e1012512 (2024).
Caicedo, A. et al. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci. Rep. 5, 9073 (2015).
Patananan, A. N. et al. Pressure-driven mitochondrial transfer pipeline generates mammalian cells of desired genetic combinations and fates. Cell Rep. 33, 108562 (2020).
Nakano, T., Nakamura, Y., Park, J.-H., Tanaka, M. & Hayakawa, K. Mitochondrial surface coating with artificial lipid membrane improves the transfer efficacy. Commun. Biol. 5, 745 (2022).
Gäbelein, C. G. et al. Mitochondria transplantation between living cells. PLoS Biol. 20, e3001576 (2022).
Zhong, Z. et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203 (2018).
Ikeda, G. et al. Mitochondria-rich extracellular vesicles from autologous stem cell–derived cardiomyocytes restore energetics of ischemic myocardium. J. Am. Coll. Cardiol. 77, 1073–1088 (2021).
Marcoux, G. et al. Platelet-derived extracellular vesicles convey mitochondrial DAMPs in platelet concentrates and their levels are associated with adverse reactions. Transfusion 59, 2403–2414 (2019).
Suh, J. et al. Mitochondrial fragmentation and donut formation enhance mitochondrial secretion to promote osteogenesis. Cell Metab. 35, 345–360.e7 (2023).
Rosina, M. et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 34, 533–548.e12 (2022).
Hayakawa, K. et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555 (2016).
van der Vlist, M. et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron 110, 613–626.e9 (2022).
Peruzzotti-Jametti, L. et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 19, e3001166 (2021).
Wu, S. et al. Extracellular vesicles meet mitochondria: potential roles in regenerative medicine. Pharmacol. Res. 206, 107307 (2024).
Acknowledgements
This work was supported by the DFG Reinhart Koselleck project (GA 2882/2-1). C.H.-L. is supported by the “T-FITNESS” Grant funded by the European Union under Grant agreement Number 101070740. J.G.B. was supported by a Fulbright Fellowship from the Australian Fulbright Commission and The Kinghorn Foundation and an Add-on Fellowship for Interdisciplinary Life Science from the Joachim Hertz Stiftung.
Author information
Authors and Affiliations
Contributions
J.G.B., C.H.-L. and L.G. conceived and wrote the manuscript. J.G.B. and C.H.-L. prepared the figures. L.G. contributed to refining the figures. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
J.G.B. and L.G. have a patent application for the use of mitochondrial transfer technology in cancer immunotherapies. L.G. has consulting agreements with Lyell Immunopharma. L.G. is a scientific advisor and a stockholder of CellRep. C.H.-L. declares no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Shikhar Mehotra and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Autophagy
-
A cellular process by which cells break down and recycle their proteins and organelles to maintain intracellular homeostasis. The specific removal of mitochondria through autophagy is known as mitophagy.
- Chimeric antigen receptor
-
(CAR). A synthetic surface receptor that typically consists of three key components: an extracellular antigen-recognition domain derived from a single-chain antibody variable fragment; an intracellular co-stimulatory domain (for example, CD28 or 4-1BB); and a CD3ζ cytoplasmic signalling domain for cell activation.
- CRISPR screen
-
A genome-wide screening approach using the CRISPR–Cas9 gene editing system in combination with libraries of guide RNAs to delete or activate large numbers of genomic loci. Cells can be selected based on phenotype, and the corresponding guide RNA that is integrated into the genome can be identified by sequencing.
- Epigenetic modifications
-
Modifications that alter gene expression and phenotype without affecting the DNA sequence. Epigenetic mechanisms include DNA methylation and modifications to histones such as methylation, acetylation or lactylation.
- Heteroplasmy
-
The presence of at least two versions of mitochondrial genomes within one cell. Different genomes may carry mutations, and microheteroplasmy (<5% mutations) is common in eukaryotic cells.
- Homoplasmy
-
Complete identity of all copies of the mitochondrial (or plastid) genome within one cell. Some tumour cells carry homoplasmically mutated mitochondrial genomes.
- Immune synapse
-
The interface between an immune cell and a target cell (for example, a cancer cell) or an antigen-presenting cell (for example, a dendritic cell, macrophage or B cell).
- Mechanistic target of rapamycin complex 1
-
(mTORC1). A multi-protein complex formed by the interaction of mTOR with DEPTOR, RPTOR, AKT1S1 and MLST8. mTORC1 is a crucial nutrient, energy and redox sensor within the cell. It regulates protein synthesis, cellular growth and metabolism by integrating signals from environmental cues such as nutrient availability and cellular energy levels.
- MitoSnap
-
A transgenic mouse model system that enables tracking of mitochondria originating from the mother cell through the permanent fluorescent labelling of a SnapSubstrate that is specifically targeted to mitochondria by SYNJ2BP. Sequential labelling of SnapTag-expressing cells with different fluorescently labelled SnapSubstrates allows for the identification and sorting of distinct cell populations based on patterns of organelle inheritance.
- Polysome
-
A polysome, or polyribosome, is a complex formed when multiple ribosomes simultaneously translate a single mRNA molecule. This arrangement allows for efficient, high-throughput protein synthesis.
- Stem-like T cells
-
Minimally differentiated T cells that share characteristics with stem cells, including self-renewal and the capacity to differentiate into various functional T cell subsets. These cells can emerge following acute infections, where they are known as stem cell memory T cells, or in response to chronic inflammation and cancer, where they are referred to as precursor exhausted T cells.
- Synthetic intramembrane proteolysis receptors
-
Engineered, Notch-based receptors activated by synthetic, bio-orthogonal or natural soluble ligands. Receptor activation depends on endocytosis and endosome acidification to elicit a cellular response.
- T cell exhaustion
-
A dysfunctional T cell state caused by chronic infection or cancer that is marked by reduced proliferation, impaired effector function, increased expression of inhibitory receptors, and distinct transcriptional and epigenetic changes.
- Tet-On system
-
A tetracycline-inducible bacteria-derived gene expression system. It consists of a reverse tetracycline transactivator that, in the drug (tetracycline)-bound state, binds to a tetracycline response element to induce expression of the downstream gene.
- Tumour-infiltrating lymphocytes
-
(TILs). The heterogeneous population of lymphocytes found in a tumour. Their relative abundance, differentiation and functions depend on type, stage and location of the cancer. T cells isolated from TILs can be activated and expanded to large numbers ex vivo before re-infusion into patients with cancer for therapeutic purposes.
- Tumour microenvironment
-
(TME). Tissue at the tumour site, consisting of cancer cells, blood vessels, immune cells and surrounding stromal cells, that, depending on the type of cancer, is often immunosuppressive and has physicochemical properties of hypoxia and low pH.
- Unfolded protein response
-
(UPR). An endoplasmic reticulum stress response triggered by the misfolding of proteins inside the cell. The response can initiate repair mechanisms to correct misfolding such as upregulating chaperones, reducing overall protein synthesis to alleviate the burden, and promoting the degradation of misfolded proteins. If these repair mechanisms fail to resolve the stress, the UPR triggers cell apoptosis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baldwin, J.G., Heuser-Loy, C. & Gattinoni, L. Targeting organelle function in T cells for cancer immunotherapy. Nat Rev Immunol (2025). https://doi.org/10.1038/s41577-025-01223-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-025-01223-9