Abstract
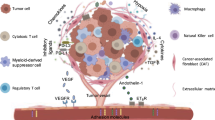
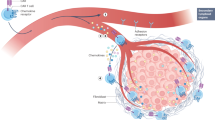
Chimeric antigen receptor (CAR) T cell therapy holds significant promise for the treatment of cancer; however, its efficacy in solid tumours is substantially hindered by the immunosuppressive tumour microenvironment (TME). Solid tumours can resist immunotherapy by impairing T cell trafficking, function and persistence. One of the initial obstacles that CAR T cells encounter is the abnormal tumour vasculature, which restricts efficient T cell infiltration, further compounded by a dense extracellular matrix. CAR T cells that do infiltrate the tumours are outnumbered by immunosuppressive cells such as regulatory T cells, myeloid-derived suppressor cells and tumour-associated macrophages. Additionally, tumour cells can contribute to CAR T cell resistance by upregulating immune checkpoint molecules, such as PDL1 and CTLA4, and engage in metabolic competition. In this Review, we discuss how cellular and non-cellular components of the TME impair CAR T cell therapy and consider potential strategies to improve CAR T cell therapies for solid tumours, either by reprogramming the TME or by engineering CAR T cells to resist the immunosuppressive effects of the TME.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Roerden, M. & Spranger, S. Cancer immune evasion, immunoediting and intratumour heterogeneity. Nat. Rev. Immunol. 25, 353–369 (2025).
Mao, X. et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol. Cancer 20, 131 (2021).
Cheng, K. et al. Tumor-associated macrophages in liver cancer: from mechanisms to therapy. Cancer Commun. 42, 1112–1140 (2022).
Propper, D. J. & Balkwill, F. R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 19, 237–253 (2022).
Ozga, A. J., Chow, M. T. & Luster, A. D. Chemokines and the immune response to cancer. Immunity 54, 859–874 (2021).
Bergers, G. & Benjamin, L. E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 3, 401–410 (2003).
Fan, Y. Vascular detransformation for cancer therapy. Trends Cancer 5, 460–463 (2019).
Huinen, Z. R., Huijbers, E. J. M., van Beijnum, J. R., Nowak-Sliwinska, P. & Griffioen, A. W. Anti-angiogenic agents—overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 18, 527–540 (2021).
Ma, W. et al. Targeting PAK4 to reprogram the vascular microenvironment and improve CAR-T immunotherapy for glioblastoma. Nat. Cancer 2, 83–97 (2021).
Huang, M. et al. c-Met-mediated endothelial plasticity drives aberrant vascularization and chemoresistance in glioblastoma. J. Clin. Invest. 126, 1801–1814 (2016).
Liu, T. et al. PDGF-mediated mesenchymal transformation renders endothelial resistance to anti-VEGF treatment in glioblastoma. Nat. Commun. 9, 3439 (2018).
Huijbers, E. J. M., Khan, K. A., Kerbel, R. S. & Griffioen, A. W. Tumors resurrect an embryonic vascular program to escape immunity. Sci. Immunol. 7, eabm6388 (2022).
Wang, Q. et al. Vascular niche IL-6 induces alternative macrophage activation in glioblastoma through HIF-2α. Nat. Commun. 9, 559 (2018).
Mulligan, J. K. & Young, M. R. Tumors induce the formation of suppressor endothelial cells in vivo. Cancer Immunol. Immunother. 59, 267–277 (2010).
Folkman, J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug. Discov. 6, 273–286 (2007).
Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005).
Huang, Y. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA 109, 17561–17566 (2012).
Dong, X. et al. Anti-VEGF therapy improves EGFR-vIII-CAR-T cell delivery and efficacy in syngeneic glioblastoma models in mice. J. Immunother. Cancer 11, e005583 (2023).
Chae, S. S. et al. Angiopoietin-2 interferes with anti-VEGFR2-induced vessel normalization and survival benefit in mice bearing gliomas. Clin. Cancer Res. 16, 3618–3627 (2010).
Park, J. S. et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell 30, 953–967 (2016).
Kim, J. et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Invest. 127, 3441–3461 (2017).
Datta, M. et al. Losartan controls immune checkpoint blocker-induced edema and improves survival in glioblastoma mouse models. Proc. Natl Acad. Sci. USA 120, e2219199120 (2023).
Maes, H. et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 26, 190–206 (2014).
Lu, Z. et al. Modified C-type natriuretic peptide normalizes tumor vasculature, reinvigorates antitumor immunity, and improves solid tumor therapies. Sci. Transl. Med. 16, eadn0904 (2024).
Cleveland, A. H. & Fan, Y. Reprogramming endothelial cells to empower cancer immunotherapy. Trends Mol. Med. 30, 126–135 (2024).
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013).
Cantelmo, A. R. et al. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 30, 968–985 (2016).
Shan, Y. et al. Targeting tumor endothelial hyperglycolysis enhances immunotherapy through remodeling tumor microenvironment. Acta Pharm. Sin. B 12, 1825–1839 (2022).
Zhang, D. et al. PHGDH-mediated endothelial metabolism drives glioblastoma resistance to chimeric antigen receptor T cell immunotherapy. Cell Metab. 35, 517–534.e8 (2023).
Schoors, S. et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 520, 192–197 (2015).
Milutinovic, S., Abe, J., Godkin, A., Stein, J. V. & Gallimore, A. The dual role of high endothelial venules in cancer progression versus immunity. Trends Cancer 7, 214–225 (2021).
Hua, Y. et al. Cancer immunotherapies transition endothelial cells into HEVs that generate TCF1+ T lymphocyte niches through a feed-forward loop. Cancer Cell 40, 1600–1618.e10 (2022).
Allen, E. et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 9, eaak9679 (2017).
Fang, W. et al. Ivonescimab plus chemotherapy in non-small cell lung cancer with EGFR variant: a randomized clinical trial. JAMA 332, 561–570 (2024).
Cox, T. R. The matrix in cancer. Nat. Rev. Cancer 21, 217–238 (2021).
Cho, Y. & Doh, J. The extracellular matrix in solid tumor immunotherapy. Trends Immunol. 45, 705–714 (2024).
Lämmermann, T. & Sixt, M. Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 21, 636–644 (2009).
Wolf, K., Müller, R., Borgmann, S., Bröcker, E. B. & Friedl, P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102, 3262–3269 (2003).
Peng, D. H. et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion. Nat. Commun. 11, 4520 (2020).
Johnston, R. J. et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 574, 565–570 (2019).
Luo, X. et al. Lymphocytes perform reverse adhesive haptotaxis mediated by LFA-1 integrins. J. Cell. Sci. 16, jcs242883 (2020).
Salmon, H. et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 122, 899–910 (2012).
He, H., Zhang, S., Tighe, S., Son, J. & Tseng, S. C. G. Immobilized heavy chain-hyaluronic acid polarizes lipopolysaccharide-activated macrophages toward M2 phenotype. J. Biol. Chem. 288, 25792–25803 (2013).
Larsen, A. M. H. et al. Collagen density modulates the immunosuppressive functions of macrophages. J. Immunol. 205, 1461–1472 (2020).
Hongu, T. et al. Perivascular tenascin C triggers sequential activation of macrophages and endothelial cells to generate a pro-metastatic vascular niche in the lungs. Nat. Cancer 3, 486–504 (2022).
Bramhall, S. R., Rosemurgy, A., Brown, P. D., Bowry, C. & Buckels, J. A. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J. Clin. Oncol. 19, 3447–3455 (2001).
Bramhall, S. R. et al. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br. J. Cancer 87, 161–167 (2002).
Goffin, J. R. et al. Phase I trial of the matrix metalloproteinase inhibitor marimastat combined with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 11, 3417–3424 (2005).
Moore, M. J. et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 21, 3296–3302 (2003).
Hingorani, S. R. et al. HALO 202: randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 36, 359–366 (2018).
McKee, T. D. et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 66, 2509–2513 (2006).
Miller, B. W. et al. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol. Med. 7, 1063–1076 (2015).
Nicolas-Boluda, A. et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. eLife 10, e58688 (2021).
Liu, J. et al. A highly selective humanized DDR1 mAb reverses immune exclusion by disrupting collagen fiber alignment in breast cancer. J. Immunother. Cancer 11, e006720 (2023).
Caruana, I. et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 21, 524–529 (2015).
Zheng, R. et al. Specific ECM degradation potentiates the antitumor activity of CAR-T cells in solid tumors. Cell Mol. Immunol. 21, 1491–1504 (2024).
Allen, G. M. & Lim, W. A. Rethinking cancer targeting strategies in the era of smart cell therapeutics. Nat. Rev. Cancer 22, 693–702 (2022).
Hamieh, M., Mansilla-Soto, J., Rivière, I. & Sadelain, M. Programming CAR T cell tumor recognition: tuned antigen sensing and logic gating. Cancer Discov. 13, 829–843 (2023).
Pines, M., Knopov, V., Genina, O., Lavelin, I. & Nagler, A. Halofuginone, a specific inhibitor of collagen type I synthesis, prevents dimethylnitrosamine-induced liver cirrhosis. J. Hepatol. 27, 391–398 (1997).
Zion, O. et al. Inhibition of transforming growth factor β signaling by halofuginone as a modality for pancreas fibrosis prevention. Pancreas 38, 427–435 (2009).
Premkumar, K. & Shankar, B. S. TGF-βR inhibitor SB431542 restores immune suppression induced by regulatory B–T cell axis and decreases tumour burden in murine fibrosarcoma. Cancer Immunol. Immunother. 70, 153–168 (2021).
Morris, J. C. et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-β (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One 9, e90353 (2014).
Takai, K., Le, A., Weaver, V. M. & Werb, Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget 7, 82889–82901 (2016).
Junttila, M. R. & de Sauvage, F. J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501, 346–354 (2013).
Kozono, S. et al. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res. 73, 2345–2356 (2013).
Froeling, F. E. et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology 141, 1486–1497.e1–e14 (2011).
McAndrews, K. M. et al. Identification of functional heterogeneity of carcinoma-associated fibroblasts with distinct IL6-mediated therapy resistance in pancreatic cancer. Cancer Discov. 12, 1580–1597 (2022).
Tran, E. et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 210, 1125–1135 (2013).
Wang, L. C. et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2, 154–166 (2014).
Liu, G., Rui, W., Zhao, X. & Lin, X. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell Mol. Immunol. 18, 1085–1095 (2021).
Beatty, G. L. et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155, 29–32 (2018).
Haas, A. R. et al. Two cases of severe pulmonary toxicity from highly active mesothelin-directed CAR T cells. Mol. Ther. 31, 2309–2325 (2023).
Togashi, Y., Shitara, K. & Nishikawa, H. Regulatory T cells in cancer immunosuppression—implications for anticancer therapy. Nat. Rev. Clin. Oncol. 16, 356–371 (2019).
Lasser, S. A., Ozbay Kurt, F. G., Arkhypov, I., Utikal, J. & Umansky, V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat. Rev. Clin. Oncol. 21, 147–164 (2024).
Blériot, C., Dunsmore, G., Alonso-Curbelo, D. & Ginhoux, F. A temporal perspective for tumor-associated macrophage identities and functions. Cancer Cell 42, 747–758 (2024).
Noy, R. & Pollard, J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014).
Cassetta, L. & Pollard, J. W. Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug. Discov. 17, 887–904 (2018).
Cinier, J. et al. Recruitment and expansion of Tregs cells in the tumor environment — how to target them? Cancers (Basel) 13, 1850 (2021).
Ghiringhelli, F. et al. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 202, 919–929 (2005).
Li, C., Jiang, P., Wei, S., Xu, X. & Wang, J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 19, 116 (2020).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Veglia, F., Perego, M. & Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 19, 108–119 (2018).
Youn, J. I., Nagaraj, S., Collazo, M. & Gabrilovich, D. I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802 (2008).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Veglia, F., Sanseviero, E. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21, 485–498 (2021).
Tanaka, A. & Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 27, 109–118 (2017).
Gambichler, T. et al. Decline of programmed death-1-positive circulating T regulatory cells predicts more favourable clinical outcome of patients with melanoma under immune checkpoint blockade. Br. J. Dermatol. 182, 1214–1220 (2020).
Duell, J. et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia 31, 2181–2190 (2017).
Casey, M. et al. Regulatory T cells hamper the efficacy of T-cell-engaging bispecific antibody therapy. Haematologica 109, 787–798 (2024).
Scholler, N. et al. Tumor immune contexture is a determinant of anti-CD19 CAR T cell efficacy in large B cell lymphoma. Nat. Med. 28, 1872–1882 (2022).
Good, Z. et al. Post-infusion CAR Treg cells identify patients resistant to CD19-CAR therapy. Nat. Med. 28, 1860–1871 (2022).
Haradhvala, N. J. et al. Distinct cellular dynamics associated with response to CAR-T therapy for refractory B cell lymphoma. Nat. Med. 28, 1848–1859 (2022).
Marshall, L. A. et al. Tumors establish resistance to immunotherapy by regulating Treg recruitment via CCR4. J. Immunother. Cancer 8, e000764 (2020).
Jackson, J. J. et al. Discovery of a potent and selective CCR4 antagonist that inhibits Treg trafficking into the tumor microenvironment. J. Med. Chem. 62, 6190–6213 (2019).
Kim, T. M. et al. Phase 2 safety and efficacy of oral CCR4 antagonist FLX475 (tivumecirnon) plus pembrolizumab in subjects with non-small cell lung cancer not previously treated with checkpoint inhibitor. J. Immunother. Cancer 11 (Suppl. 2), Abstract 629-C (2023).
Muzaffar, J. et al. Abstract CT226: phase 2 study of oral CCR4 antagonist FLX475 (tivumecirnon) plus pembrolizumab in subjects with head and neck squamous cell carcinoma (HNSCC) previously treated with checkpoint inhibitor. Cancer Res. 84, CT226 (2024).
Hoelzinger, D. B. et al. Blockade of CCL1 inhibits T regulatory cell suppressive function enhancing tumor immunity without affecting T effector responses. J. Immunol. 184, 6833–6842 (2010).
Villarreal, D. O. et al. Targeting CCR8 induces protective antitumor immunity and enhances vaccine-induced responses in colon cancer. Cancer Res. 78, 5340–5348 (2018).
Kim, N. et al. CCR8 as a therapeutic novel target: omics-integrated comprehensive analysis for systematically prioritizing indications. Biomedicines 11, 2910 (2023).
Roider, H. G. et al. Selective depletion of tumor-infiltrating regulatory T cells with BAY 3375968, a novel Fc-optimized anti-CCR8 antibody. Clin. Exp. Med. 24, 122 (2024).
Tan, S. N. et al. Regulatory T cells converted from TH1 cells in tumors suppress cancer immunity via CD39. J. Exp. Med. 222, e20240445(2025).
Shimizu, J., Yamazaki, S. & Sakaguchi, S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163, 5211–5218 (1999).
Onizuka, S. et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 59, 3128–3133 (1999).
Zammarchi, F. et al. CD25-targeted antibody–drug conjugate depletes regulatory T cells and eliminates established syngeneic tumors via antitumor immunity. J. Immunother. Cancer 8, e000860 (2020).
Powell, D. J. Jr. et al. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J. Immunol. 179, 4919–4928 (2007).
Onda, M., Kobayashi, K. & Pastan, I. Depletion of regulatory T cells in tumors with an anti-CD25 immunotoxin induces CD8 T cell-mediated systemic antitumor immunity. Proc. Natl Acad. Sci. USA 116, 4575–4582 (2019).
Selby, M. J. et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 1, 32–42 (2013).
Tanaka, A. & Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 49, 1140–1146 (2019).
Ha, D. et al. Differential control of human Treg and effector T cells in tumor immunity by Fc-engineered anti-CTLA-4 antibody. Proc. Natl Acad. Sci. USA 116, 609–618 (2019).
Arce Vargas, F. et al. Fc-optimized anti-CD25 depletes tumor-infiltrating regulatory T cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity 46, 577–586 (2017).
Solomon, I. et al. CD25-Treg-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat. Cancer 1, 1153–1166 (2020).
Long, X. et al. Targeting JMJD1C to selectively disrupt tumor Treg cell fitness enhances antitumor immunity. Nat. Immunol. 25, 525–536 (2024).
Tanaka, A. et al. Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. J. Exp. Med. 217, e20191009 (2020).
Sun, Y. et al. Depletion of Tregs from CD4+ CAR-T cells enhances the tumoricidal effect of CD8+ CAR-T cells in anti-CD19 CAR-T therapy. FEBS J. 292, 1904–1919 (2024).
Wellhausen, N. et al. Epitope base editing CD45 in hematopoietic cells enables universal blood cancer immune therapy. Sci. Transl. Med. 15, eadi1145 (2023).
Wellhausen, N., Baek, J., Gill, S. I. & June, C. H. Enhancing cellular immunotherapies in cancer by engineering selective therapeutic resistance. Nat. Rev. Can. 24, 614–628 (2024).
Pan, J. et al. Allogeneic CD5-specific CAR-T therapy for relapsed/refractory T-ALL: a phase 1 trial. Nat. Med. 31, 126–136 (2025).
Oh, B. L. Z. et al. Fratricide-resistant CD7-CAR T cells in T-ALL. Nat. Med. 30, 3687–3696 (2024).
Lozano, T. et al. Searching for peptide inhibitors of T regulatory cell activity by targeting specific domains of FOXP3 transcription factor. Biomedicines 9, 197 (2021).
Manrique-Rincón, A. J. et al. Aptamer-mediated transcriptional gene silencing of Fox p 3 inhibits regulatory T cells and potentiates antitumor response. Mol. Ther. Nucleic Acids 25, 143–151 (2021).
Kofler, D. M. et al. CD28 costimulation impairs the efficacy of a redirected T-cell antitumor attack in the presence of regulatory T cells which can be overcome by preventing Lck activation. Mol. Ther. 19, 760–767 (2011).
Suryadevara, C. M. et al. Preventing Lck activation in CAR T cells confers Treg resistance but requires 4-1BB signaling for them to persist and treat solid tumors in nonlymphodepleted hosts. Clin. Cancer Res. 25, 358–368 (2019).
Zhang, Q. et al. A human orthogonal IL-2 and IL-2Rβ system enhances CAR T cell expansion and antitumor activity in a murine model of leukemia. Sci. Transl. Med. 13, eabg6986 (2021).
Golumba-Nagy, V., Kuehle, J., Hombach, A. A. & Abken, H. CD28-ζ CAR T cells resist TGF-β repression through IL-2 signaling, which can be mimicked by an engineered IL-7 autocrine loop. Mol. Ther. 26, 2218–2230 (2018).
Kalbasi, A. et al. Potentiating adoptive cell therapy using synthetic IL-9 receptors. Nature 607, 360–365 (2022).
Pezzella, M. et al. Tumor-derived G-CSF induces an immunosuppressive microenvironment in an osteosarcoma model, reducing response to CAR.GD2 T-cells. J. Hematol. Oncol. 17, 127 (2024).
Zhu, J. et al. Elevated CD10– G-MDSC-like neutrophils correlate with non-response and poor prognosis of CD19 CAR T-cell therapy for B-cell acute lymphoblastic leukemia. Blood 144, 7163 (2024).
Galli, E. et al. High levels of circulating granulocytic myeloid-derived suppressor cells (G-MDSCs) predict failure of CD19-targeting CAR-T cell therapy. Blood 142, 1015 (2023).
Bullock, K. & Richmond, A. Suppressing MDSC recruitment to the tumor microenvironment by antagonizing CXCR2 to enhance the efficacy of immunotherapy. Cancers (Basel) 13, 6293 (2021).
Dang, Y. et al. GOLM1 drives colorectal cancer metastasis by regulating myeloid-derived suppressor cells. J. Cancer 12, 7158–7166 (2021).
Flores-Toro, J. A. et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc. Natl Acad. Sci. USA 117, 1129–1138 (2020).
Yang, L. et al. Blockade of CCR5-mediated myeloid derived suppressor cell accumulation enhances anti-PD1 efficacy in gastric cancer. Immunopharmacol. Immunotoxicol. 40, 91–97 (2018).
Lu, Z. et al. Epigenetic therapy inhibits metastases by disrupting premetastatic niches. Nature 579, 284–290 (2020).
Zhang, B. et al. Optimized CAR-T therapy based on spatiotemporal changes and chemotactic mechanisms of MDSCs induced by hypofractionated radiotherapy. Mol. Ther. 31, 2105–2119 (2023).
Sun, R. et al. Olaparib suppresses MDSC recruitment via SDF1α/CXCR4 axis to improve the anti-tumor efficacy of CAR-T cells on breast cancer in mice. Mol. Ther. 29, 60–74 (2021).
Xiao, Z. et al. Desmoplastic stroma restricts T cell extravasation and mediates immune exclusion and immunosuppression in solid tumors. Nat. Commun. 14, 5110 (2023).
Vincent, J. et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061 (2010).
Suzuki, E., Kapoor, V., Jassar, A. S., Kaiser, L. R. & Albelda, S. M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721 (2005).
Huang, X., Cui, S. & Shu, Y. Cisplatin selectively downregulated the frequency and immunoinhibitory function of myeloid-derived suppressor cells in a murine B16 melanoma model. Immunol. Res. 64, 160–170 (2016).
Zheng, Y. et al. Cimetidine suppresses lung tumor growth in mice through proapoptosis of myeloid-derived suppressor cells. Mol. Immunol. 54, 74–83 (2013).
Vila-Leahey, A. et al. Ranitidine modifies myeloid cell populations and inhibits breast tumor development and spread in mice. Oncoimmunology 5, e1151591 (2016).
Fultang, L. et al. MDSC targeting with gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers. EBioMedicine 47, 235–246 (2019).
Dominguez, G. A. et al. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin. Cancer Res. 23, 2942–2950 (2017).
Nalawade, S. A. et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J. Immunother. Cancer 9, e003237 (2021).
Parihar, R. et al. NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol. Res. 7, 363–375 (2019).
Li, H. et al. CAIX-specific CAR-T cells and sunitinib show synergistic effects against metastatic renal cancer models. J. Immunother. 43, 16–28 (2020).
Lu, M. et al. Lenvatinib enhances T cell immunity and the efficacy of adoptive chimeric antigen receptor-modified T cells by decreasing myeloid-derived suppressor cells in cancer. Pharmacol. Res. 174, 105829 (2021).
Serafini, P. et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 203, 2691–2702 (2006).
Nagaraj, S. et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin. Cancer Res. 16, 1812–1823 (2010).
Hossain, F. et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol. Res. 3, 1236–1247 (2015).
Veglia, F. et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 569, 73–78 (2019).
Nefedova, Y. et al. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 67, 11021–11028 (2007).
Mirza, N. et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 66, 9299–9307 (2006).
Tobin, R. P. et al. Targeting MDSC differentiation using ATRA: a phase I/II clinical trial combining pembrolizumab and all-trans retinoic acid for metastatic melanoma. Clin. Cancer Res. 29, 1209–1219 (2023).
Long, A. H. et al. Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunol. Res. 4, 869–880 (2016).
Frost, B. F. et al. BTK inhibition prevents the induction of myeloid-derived suppressor cells in malignancy and improves CART function. Blood 144, 909 (2024).
Xu, M., Wang, Y., Xia, R., Wei, Y. & Wei, X. Role of the CCL2–CCR2 signalling axis in cancer: mechanisms and therapeutic targeting. Cell Prolif. 54, e13115 (2021).
Kloosterman, D. J. & Akkari, L. Macrophages at the interface of the co-evolving cancer ecosystem. Cell 186, 1627–1651 (2023).
Takenaka, M. C. et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 22, 729–740 (2019).
Li, X. et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 66, 157–167 (2017).
Wang, Y. et al. mRNA delivery of a bispecific single-domain antibody to polarize tumor-associated macrophages and synergize immunotherapy against liver malignancies. Adv. Mater. 33, e2007603 (2021).
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013).
Butowski, N. et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 18, 557–564 (2016).
Manji, G. A. et al. A phase I study of the combination of pexidartinib and sirolimus to target tumor-associated macrophages in unresectable sarcoma and malignant peripheral nerve sheath tumors. Clin. Cancer Res. 27, 5519–5527 (2021).
Rao, R. et al. Glioblastoma genetic drivers dictate the function of tumor-associated macrophages/microglia and responses to CSF1R inhibition. Neuro Oncol. 24, 584–597 (2022).
Rodriguez-Garcia, A. et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat. Commun. 12, 877 (2021).
Sánchez-Paulete, A. R. et al. Targeting macrophages with CAR T cells delays solid tumor progression and enhances antitumor immunity. Cancer Immunol. Res. 10, 1354–1369 (2022).
Scott, E. M. et al. Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples. J. Immunother. Cancer 7, 320 (2019).
Beatty, G. L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011).
Stromnes, I. M. et al. Differential effects of depleting versus programming tumor-associated macrophages on engineered T cells in pancreatic ductal adenocarcinoma. Cancer Immunol. Res. 7, 977–989 (2019).
Winograd, R. et al. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol. Res. 3, 399–411 (2015).
Zeng, Q. & Jewell, C. M. Directing toll-like receptor signaling in macrophages to enhance tumor immunotherapy. Curr. Opin. Biotechnol. 60, 138–145 (2019).
Rodell, C. B. et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2, 578–588 (2018).
Quail, D. F. et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, aad3018 (2016).
Poh, A. R. et al. Therapeutic inhibition of the SRC-kinase HCK facilitates T cell tumor infiltration and improves response to immunotherapy. Sci. Adv. 8, eabl7882 (2022).
Yang, F. et al. Small-molecule toosendanin reverses macrophage-mediated immunosuppression to overcome glioblastoma resistance to immunotherapy. Sci. Transl. Med. 15, eabq3558 (2023).
Yamada-Hunter, S. A. et al. Engineered CD47 protects T cells for enhanced antitumour immunity. Nature 630, 457–465 (2024).
Dacek, M. M. et al. Potentiating antibody-dependent killing of cancers with CAR T cells secreting CD47-SIRPα checkpoint blocker. Blood 141, 2003–2015 (2023).
Kagoya, Y. Cytokine signaling in chimeric antigen receptor T-cell therapy. Int. Immunol. 36, 49–56 (2024).
Bell, M. & Gottschalk, S. Engineered cytokine signaling to improve CAR T cell effector function. Front. Immunol. 12, 684642 (2021).
Svoboda, J. et al. Enhanced CAR T-cell therapy for lymphoma after previous failure. N. Engl. J. Med. 392, 1824–1835 (2025).
Cadilha, B. L. et al. Combined tumor-directed recruitment and protection from immune suppression enable CAR T cell efficacy in solid tumors. Sci. Adv. 7, eabi5781 (2021).
Kloss, C. C. et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 26, 1855–1866 (2018).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
Noh, K. E. et al. TGF-β/IL-7 chimeric switch receptor-expressing CAR-T cells inhibit recurrence of CD19-positive B cell lymphoma. Int. J. Mol. Sci. 22, 8706 (2021).
Roth, T. L. et al. Pooled knockin targeting for genome engineering of cellular immunotherapies. Cell 181, 728–744.e21 (2020).
Mohammed, S. et al. Improving chimeric antigen receptor-modified T cell function by reversing the immunosuppressive tumor microenvironment of pancreatic cancer. Mol. Ther. 25, 249–258 (2017).
Wilkie, S. et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J. Biol. Chem. 285, 25538–25544 (2010).
Tang, N. et al. TGF-β inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight 5, e133977 (2020).
Travis, M. A. & Sheppard, D. TGF-β activation and function in immunity. Annu. Rev. Immunol. 32, 51–82 (2014).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018).
Cherkassky, L. et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 126, 3130–3144 (2016).
Chong, E. A. et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood 129, 1039–1041 (2017).
Heczey, A. et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 25, 2214–2224 (2017).
Rafiq, S. et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 36, 847–856 (2018).
Li, S. et al. Enhanced cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors. Clin. Cancer Res. 23, 6982–6992 (2017).
Prosser, M. E., Brown, C. E., Shami, A. F., Forman, S. J. & Jensen, M. C. Tumor PD-L1 co-stimulates primary human CD8+ cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol. Immunol. 51, 263–272 (2012).
Liu, X. et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 76, 1578–1590 (2016).
James, S. E. et al. Leucine zipper-based immunomagnetic purification of CAR T cells displaying multiple receptors. Nat. Biomed. Eng. 8, 1592–1614 (2024).
Qin, L. et al. Co-expression of a PD-L1-specific chimeric switch receptor augments the efficacy and persistence of CAR T cells via the CD70–CD27 axis. Nat. Commun. 13, 6051 (2022).
Rupp, L. J. et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 7, 737 (2017).
Lee, Y. H. et al. PD-1 and TIGIT downregulation distinctly affect the effector and early memory phenotypes of CD19-targeting CAR T cells. Mol. Ther. 30, 579–592 (2022).
Choi, B. D. et al. CRISPR–Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J. Immunother. Cancer 7, 304 (2019).
Agarwal, S. et al. Deletion of the inhibitory co-receptor CTLA-4 enhances and invigorates chimeric antigen receptor T cells. Immunity 56, 2388–2407.e9 (2023).
Grosser, R., Cherkassky, L., Chintala, N. & Adusumilli, P. S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 36, 471–482 (2019).
Lyssiotis, C. A. & Kimmelman, A. C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 27, 863–875 (2017).
Rial Saborido, J., Völkl, S., Aigner, M., Mackensen, A. & Mougiakakos, D. Role of CAR T cell metabolism for therapeutic efficacy. Cancers (Basel) 14, 5442 (2022).
Foeng, J., Comerford, I. & McColl, S. R. Harnessing the chemokine system to home CAR-T cells into solid tumors. Cell Rep. Med. 3, 100543 (2022).
Whilding, L. M. et al. CAR T-cells targeting the integrin αvβ6 and co-expressing the chemokine receptor CXCR2 demonstrate enhanced homing and efficacy against several solid malignancies. Cancers (Basel) 11, 674 (2019).
Liu, G. et al. CXCR2-modified CAR-T cells have enhanced trafficking ability that improves treatment of hepatocellular carcinoma. Eur. J. Immunol. 50, 712–724 (2020).
Dai, Z. et al. Ectopic CXCR2 expression cells improve the anti-tumor efficiency of CAR-T cells and remodel the immune microenvironment of pancreatic ductal adenocarcinoma. Cancer Immunol. Immunother. 73, 61 (2024).
Johnston, A. C. et al. Engineering self-propelled tumor-infiltrating CAR T cells using synthetic velocity receptors. Preprint at bioRxiv https://doi.org/10.1101/2023.12.13.571595 (2024).
Flugel, C. L. et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 20, 49–62 (2023).
Juillerat, A. et al. An oxygen sensitive self-decision making engineered CAR T-cell. Sci. Rep. 7, 39833 (2017).
Vogt, K. C. et al. Microenvironment actuated CAR T cells improve solid tumor efficacy without toxicity. Sci. Adv. 11, eads3403 (2025).
Simic, M. S. et al. Programming tissue-sensing T cells that deliver therapies to the brain. Science 386, eadl4237 (2024).
Tieu, V. et al. A versatile CRISPR–Cas13d platform for multiplexed transcriptomic regulation and metabolic engineering in primary human T cells. Cell 187, 1278–1295.e20 (2024).
Diorio, C. et al. Cytosine base editing enables quadruple-edited allogeneic CART cells for T-ALL. Blood 140, 619–629 (2022).
Herrera, F. G., Bourhis, J. & Coukos, G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 67, 65–85 (2017).
Hauth, F., Ho, A. Y., Ferrone, S. & Duda, D. G. Radiotherapy to enhance chimeric antigen receptor T-cell therapeutic efficacy in solid tumors: a narrative review. JAMA Oncol. 7, 1051–1059 (2021).
Rodríguez-Ruiz, M. E., Vanpouille-Box, C., Melero, I., Formenti, S. C. & Demaria, S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 39, 644–655 (2018).
Herrera, F. G. et al. Low-dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov. 12, 108–133 (2022).
Ni, H. et al. FLASH radiation reprograms lipid metabolism and macrophage immunity and sensitizes medulloblastoma to CAR-T cell therapy. Nat. Cancer 6, 460–473 (2025).
Mai, D. et al. Combined disruption of T cell inflammatory regulators regnase-1 and roquin-1 enhances antitumor activity of engineered human T cells. Proc. Natl Acad. Sci. USA 120, e2218632120 (2023).
Uslu, U. et al. Chimeric antigen receptor T cells as adjuvant therapy for unresectable adenocarcinoma. Sci. Adv. 9, eade2526 (2023).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830.e14 (2018).
Lin, H. et al. Understanding the immunosuppressive microenvironment of glioma: mechanistic insights and clinical perspectives. J. Hematol. Oncol. 17, 31 (2024).
Zhou, Z. et al. Deciphering the tumor immune microenvironment from a multidimensional omics perspective: insight into next-generation CAR-T cell immunotherapy and beyond. Mol. Cancer 23, 131 (2024).
Patnaik, A. et al. Preliminary results of a phase 1, first-in-human, dose escalation study of the anti-CCR8 cytolytic antibody, CHS-114 (formerly SRF114) in patients with advanced solid tumors. J. Clin. Oncol. 42, 2664–2664 (2024).
Wei, X. et al. A CD25 × TIGIT bispecific antibody induces anti-tumor activity through selective intratumoral Treg cell depletion. Mol. Ther. 32, 4075–4094 (2024).
Yoshida, T. et al. C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J. Immunother. Cancer 8, e000234 (2020).
Geyer, M. B. et al. CD371-targeted CAR T-cells secreting interleukin-18 exhibit robust expansion and disease clearance in patients with refractory acute myeloid leukemia. Blood 144, 2070 (2024).
Zhao, Y. et al. IL-10-expressing CAR T cells resist dysfunction and mediate durable clearance of solid tumors and metastases. Nat. Biotechnol. 42, 1693–1704 (2024).
Guo, Y. et al. IL-10 expressing CD19 CAR-T cells induce complete remission and improve long-term protection in relapsed or refractory B-cell hematological malignancies. Blood 144, 92 (2024).
Carlini, V. et al. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 14, 1161067 (2023).
Lei, W. et al. Safety and feasibility of anti-CD19 CAR T cells expressing inducible IL-7 and CCL19 in patients with relapsed or refractory large B-cell lymphoma. Cell Discov. 10, 5 (2024).
Jain, M. D. et al. Five-year follow-up of standard-of-care axicabtagene ciloleucel for large B-cell lymphoma: results from the US lymphoma CAR T consortium. J. Clin. Oncol. 42, 3581–3592 (2024).
Sesques, P. et al. Novel prognostic scoring systems for severe CRS and ICANS after anti-CD19 CAR T cells in large B-cell lymphoma. J. Hematol. Oncol. 17, 61 (2024).
Steffin, D. et al. Interleukin-15-armoured GPC3 CAR T cells for patients with solid cancers. Nature 637, 940–946 (2025).
O’Cearbhaill, R. E. et al. A phase I clinical trial of autologous chimeric antigen receptor (CAR) T cells genetically engineered to secrete IL-12 and to target the MUC16ecto antigen in patients (pts) with MUC16ecto+ recurrent high-grade serous ovarian cancer (HGSOC). Gynecol. Oncol. 159, 42 (2020).
Chmielewski, M., Hombach, A. A. & Abken, H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol. Rev. 257, 83–90 (2014).
Chmielewski, M., Kopecky, C., Hombach, A. A. & Abken, H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 71, 5697–5706 (2011).
Agliardi, G. et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat. Commun. 12, 444 (2021).
Levine, B. L., Miskin, J., Wonnacott, K. & Keir, C. Global manufacturing of CAR T cell therapy. Mol. Ther.-Methods Clin. Dev. 4, 92–101 (2017).
Ghassemi, S. et al. Rapid manufacturing of non-activated potent CAR T cells. Nat. Biomed. Eng. 6, 118–128 (2022).
Dickinson, M. J. et al. A novel autologous CAR-T therapy, YTB323, with preserved T-cell stemness shows enhanced CAR T-cell efficacy in preclinical and early clinical development. Cancer Discov. 13, 1982–1997 (2023).
Shen, L. et al. Metabolic reprogramming by ex vivo glutamine inhibition endows CAR-T cells with less-differentiated phenotype and persistent antitumor activity. Cancer Lett. 538, 215710 (2022).
Cappabianca, D. et al. Metabolic priming of GD2 TRAC-CAR T cells during manufacturing promotes memory phenotypes while enhancing persistence. Mol. Ther. Methods Clin. Dev. 32, 101249 (2024).
Klein Geltink, R. I. et al. Metabolic conditioning of CD8+ effector T cells for adoptive cell therapy. Nat. Metab. 2, 703–716 (2020).
Frisch, A. T. et al. Redirecting glucose flux during in vitro expansion generates epigenetically and metabolically superior T cells for cancer immunotherapy. Cell Metab. 37, 870–885 (2025).
Nava Lauson, C. B. et al. Linoleic acid potentiates CD8+ T cell metabolic fitness and antitumor immunity. Cell Metab. 35, 633–650.e9 (2023).
Luu, M. et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 12, 4077 (2021).
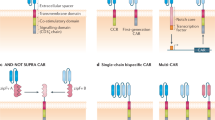
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Lakhani, A. et al. Extracellular domains of CARs reprogramme T cell metabolism without antigen stimulation. Nat. Metab. 6, 1143–1160 (2024).
Ajina, A. & Maher, J. Strategies to address chimeric antigen receptor tonic signaling. Mol. Cancer Ther. 17, 1795–1815 (2018).
Singh, N. et al. Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells. Nat. Med. 27, 842–850 (2021).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Razavi, A. S., Loskog, A., Razi, S. & Rezaei, N. The signaling and the metabolic differences of various CAR T cell designs. Int. Immunopharmacol. 114, 109593 (2023).
Guerrero, J. A. et al. GLUT1 overexpression in CAR-T cells induces metabolic reprogramming and enhances potency. Nat. Commun. 15, 8658 (2024).
Shi, Y. et al. GLUT1 overexpression enhances CAR T cell metabolic fitness and anti-tumor efficacy. Mol. Ther. 32, 2393–2405 (2024).
Valentić, B. et al. The glucose transporter 5 enhances CAR-T cell metabolic function and anti-tumour durability. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-4342820/v1 (2024).
Panetti, S. et al. Engineering amino acid uptake or catabolism promotes CAR T-cell adaption to the tumor environment. Blood Adv. 7, 1754–1761 (2023).
Hwang, S. M. et al. Transgelin 2 guards T cell lipid metabolism and antitumour function. Nature 635, 1010–1018 (2024).
Xu, S. et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity 54, 1561–1577.e7 (2021).
Peralta, R. M. et al. Dysfunction of exhausted T cells is enforced by MCT11-mediated lactate metabolism. Nat. Immunol. 25, 2297–2307 (2024).
Quinn, W. J. et al. Lactate limits T cell proliferation via the NAD(H) redox state. Cell Rep. 33, 108500 (2020).
Notarangelo, G. et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8+ T cell function. Science 377, 1519–1529 (2022).
Cheng, J. et al. Cancer-cell-derived fumarate suppresses the anti-tumor capacity of CD8+ T cells in the tumor microenvironment. Cell Metab. 35, 961–978.e10 (2023).
Ligtenberg, M. A. et al. Coexpressed catalase protects chimeric antigen receptor-redirected T cells as well as bystander cells from oxidative stress-induced loss of antitumor activity. J. Immunol. 196, 759–766 (2016).
Pilipow, K. et al. Antioxidant metabolism regulates CD8+ T memory stem cell formation and antitumor immunity. JCI Insight 3, e122299 (2018).
Varanasi, S. K. et al. Bile acid synthesis impedes tumor-specific T cell responses during liver cancer. Science 387, 192–201 (2025).
Fultang, L. et al. Metabolic engineering against the arginine microenvironment enhances CAR-T cell proliferation and therapeutic activity. Blood 136, 1155–1160 (2020).
Steggerda, S. M. et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J. Immunother. Cancer 5, 101 (2017).
Martí i Líndez, A. A. et al. Mitochondrial arginase-2 is a cell-autonomous regulator of CD8+ T cell function and antitumor efficacy. JCI Insight 4, e132975 (2019).
Ninomiya, S. et al. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood 125, 3905–3916 (2015).
Li, P. et al. IDO inhibition facilitates antitumor immunity of Vγ9Vδ2 T cells in triple-negative breast cancer. Front. Oncol. 11, 679517 (2021).
Huang, Q. et al. miR-153 suppresses IDO1 expression and enhances CAR T cell immunotherapy. J. Hematol. Oncol. 11, 58 (2018).
Shao, J. et al. Indoleamine 2,3-dioxygenase 1 inhibitor-loaded nanosheets enhance CAR-T cell function in esophageal squamous cell carcinoma. Front. Immunol. 12, 661357 (2021).
Ye, L. et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab. 34, 595–614.e14 (2022).
Minogue, E. et al. Glutarate regulates T cell metabolism and anti-tumour immunity. Nat. Metab. 5, 1747–1764 (2023).
Chan, J. D. et al. FOXO1 enhances CAR T cell stemness, metabolic fitness and efficacy. Nature 629, 201–210 (2024).
Lontos, K. et al. Metabolic reprogramming via an engineered PGC-1α improves human chimeric antigen receptor T-cell therapy against solid tumors. J. Immunother. Cancer 11, e006522 (2023).
Doan, A. E. et al. FOXO1 is a master regulator of memory programming in CAR T cells. Nature 629, 211–218 (2024).
Hatae, R. et al. Enhancing CAR-T cell metabolism to overcome hypoxic conditions in the brain tumor microenvironment. JCI Insight 9, e177141 (2024).
Acknowledgements
This work was supported, in part, by National Institutes of Health (NIH) grants R01NS094533 (to Y.F.), R01NS106108 (to Y.F.), R01CA241501 (to Y.F.) and R01HL155198 (to Y.F.), and American Cancer Society Yosemite Award 24-1331395 (to Y.F.).
Author information
Authors and Affiliations
Contributions
Z.L.L. and N.W. contributed equally to all aspects of the Review; generated all figures, boxes and table; and wrote the original version of the Review. C.H.J. and Y.F. edited the Review and provided supervision.
Corresponding authors
Ethics declarations
Competing interests
C.H.J. is an inventor of patents and/or patent applications licensed to Novartis Institutes of Biomedical Research and receives licence revenue from such licences; is an inventor on patents and/or patent applications licensed to Kite Pharma, Capstan Therapeutics, Dispatch Therapeutics and BlueWhale Bio; and is a member of the scientific advisory boards of AC Immune, BluesphereBio, BlueWhale Bio, Cabaletta, Carisma, Cartography, Cellares, Celldex, Decheng, Replay Bio, Verismo and WIRB-Copernicus. Y.F. is a co-founder of Radix Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Marcela Maus and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lamplugh, Z.L., Wellhausen, N., June, C.H. et al. Microenvironmental regulation of solid tumour resistance to CAR T cell therapy. Nat Rev Immunol (2025). https://doi.org/10.1038/s41577-025-01229-3
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41577-025-01229-3