Abstract
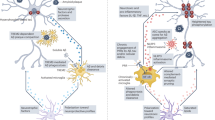
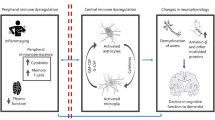
Dysregulation of the immune system is a cardinal feature of Alzheimer disease (AD), and a considerable body of evidence indicates pathological alterations in central and peripheral immune responses that change over time. Considering AD as a systemic immune process raises important questions about how communication between the peripheral and central compartments occurs and whether this crosstalk represents a therapeutic target. We established a whitepaper workgroup to delineate the current status of the field and to outline a research prospectus for advancing our understanding of peripheral–central immune crosstalk in AD. To guide the prospectus, we begin with an overview of seminal clinical observations that suggest a role for peripheral immune dysregulation and peripheral–central immune communication in AD, followed by formative animal data that provide insights into possible mechanisms for these clinical findings. We then present a roadmap that defines important next steps needed to overcome conceptual and methodological challenges, opportunities for future interdisciplinary research, and suggestions for translating promising mechanistic studies into therapeutic interventions.
Key points
-
Alzheimer disease (AD) should be viewed as a systemic disease that involves dynamic processes in the peripheral and central immune compartments.
-
Clinical studies suggest that the peripheral and central immune systems are dysregulated in AD, are related to cognitive function and clinical status, and may change in a non-linear manner over time; burgeoning evidence also suggests that the roles of innate and adaptive immune processes differ depending on the pathological stage of AD.
-
Animal studies have provided insights into possible mechanisms for peripheral and central immune communication, including direct pathways that involve peripheral immune cell infiltration into the CNS, as well as indirect pathways that involve systemic inflammation-driven modulation of microglial function.
-
Longitudinal clinical studies to evaluate peripheral and central immune mechanisms are needed in combination with comprehensive assessment of the human exposome in demographically diverse cohorts.
-
Further mechanistic studies in animal models are critical to providing a better understanding of peripheral immune cell trafficking to the CNS, adaptive and innate immune system interfaces and astrocyte–microglia crosstalk.
-
Translational and interdisciplinary studies are needed to reconcile differences in immune pathways across species and to develop strategic immune-based therapies that are tailored to disease state and severity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Change history
08 October 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41582-021-00579-5
References
Paouri, E. & Georgopoulos, S. Systemic and CNS inflammation crosstalk: implications for Alzheimer’s disease. Curr. Alzheimer Res. 16, 559–574 (2019).
Morris, G., Berk, M., Maes, M. & Puri, B. K. Could Alzheimer’s disease originate in the periphery and if so how so? Mol. Neurobiol. 56, 406–434 (2019).
Stephenson, J., Nutma, E., van der Valk, P. & Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 154, 204–219 (2018).
Webers, A., Heneka, M. T. & Gleeson, P. A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 98, 28–41 (2020).
Kinney, J. W. et al. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 4, 575–590 (2018).
Le Page, A. et al. Role of the peripheral innate immune system in the development of Alzheimer’s disease. Exp. Gerontol. 107, 59–66 (2018).
Jenny, N. S. et al. Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. J. Gerontol. A Biol. Sci. Med. Sci. 67, 970–976 (2012).
Metti, A. L. et al. Change in inflammatory markers and cognitive status in the oldest-old women from the Study of Osteoporotic Fractures. J. Am. Geriatrics Soc. 62, 662–666 (2014).
Sundelof, J. et al. Systemic inflammation and the risk of Alzheimer’s disease and dementia: a prospective population-based study. J. Alzheimers Dis. 18, 79–87 (2009).
Lanzrein, A. S. et al. Longitudinal study of inflammatory factors in serum, cerebrospinal fluid, and brain tissue in Alzheimer disease: interleukin-1β, interleukin-6, interleukin-1 receptor antagonist, tumor necrosis factor-a, the soluble tumor necrosis factor receptors I and II, and α1-antichymotrypsin. Alzheimer Dis. Associated Disord. 12, 215–227 (1998).
Ravaglia, G. et al. Blood inflammatory markers and risk of dementia: the Conselice Study of Brain Aging. Neurobiol. Aging 28, 1810–1820 (2007).
Eriksson, U. K. et al. Associations of gene sequence variation and serum levels of C-reactive protein and interleukin-6 with Alzheimer’s disease and dementia. J. Alzheimers Dis. 23, 361–369 (2011).
Swardfager, W. et al. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 68, 930–941 (2010).
Lai, K. S. P. et al. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry 88, 876–882 (2017).
Darweesh, S. K. L. et al. Inflammatory markers and the risk of dementia and Alzheimer’s disease: a meta-analysis. Alzheimers Dement. 14, 1450–1459 (2018).
Koyama, A. et al. The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: a meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 68, 433–440 (2013).
Schmidt, R. et al. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann. Neurol. 52, 168–174 (2002).
Engelhart, M. J. et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam Study. Arch. Neurol. 61, 668–672 (2004).
Tan, Z. S. et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology 68, 1902–1908 (2007).
Weaver, J. D. et al. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 59, 371–378 (2002).
Yaffe, K. et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 61, 76–80 (2003).
Singh-Manoux, A. et al. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology 83, 486–493 (2014).
Yaffe, K. et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292, 2237–2242 (2004).
Tao, Q. et al. Association of chronic low-grade inflammation with risk of Alzheimer disease in ApoE4 carriers. JAMA Netw. Open 1, e183597 (2018).
Walker, K. A. et al. Systemic inflammation during midlife and cognitive change over 20 years: the ARIC Study. Neurology 92, e1256–e1267 (2019).
Gottesman, R. F. et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 74, 1246–1254 (2017).
Bettcher, B. M. et al. Increases in a pro-inflammatory chemokine, MCP-1, are related to decreases in memory over time. Front. Aging Neurosci. 11, 25 (2019).
Marsland, A. L. et al. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom. Med. 68, 895–903 (2006).
Wersching, H. et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 74, 1022–1029 (2010).
Jefferson, A. L. et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology 68, 1032–1038 (2007).
Bettcher, B. M. et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav. Immun. 26, 103–108 (2012).
Walker, K. A. et al. Association of peripheral inflammatory markers with connectivity in large-scale functional brain networks of non-demented older adults. Brain Behav. Immun. 87, 388–396 (2020).
Walker, K. A. et al. The association of mid- and late-life systemic inflammation with brain amyloid deposition: the ARIC-PET Study. J. Alzheimers Dis. 66, 1041–1052 (2018).
Shen, X. N. et al. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: a meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry 90, 590–598 (2019).
Diniz, B. S. et al. Higher serum sTNFR1 level predicts conversion from mild cognitive impairment to Alzheimer’s disease. J. Alzheimers Dis. 22, 1305–1311 (2010).
Saleem, M., Herrmann, N., Swardfager, W., Eisen, R. & Lanctot, K. L. Inflammatory markers in mild cognitive impairment: a meta-analysis. J. Alzheimers Dis. 47, 669–679 (2015).
Leung, R. et al. Inflammatory proteins in plasma are associated with severity of Alzheimer’s disease. PLoS ONE 8, e64971 (2013).
Galimberti, D. et al. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol. Aging 27, 1763–1768 (2006).
Morgan, A. R. et al. Inflammatory biomarkers in Alzheimer’s disease plasma. Alzheimers Dement. 15, 776–787 (2019).
Motta, M., Imbesi, R., Di Rosa, M., Stivala, F. & Malaguarnera, L. Altered plasma cytokine levels in Alzheimer’s disease: correlation with the disease progression. Immunol. Lett. 114, 46–51 (2007).
Brosseron, F., Krauthausen, M., Kummer, M. & Heneka, M. T. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol. Neurobiol. 50, 534–544 (2014).
Riphagen, J. M. et al. Linking APOE-epsilon4, blood-brain barrier dysfunction, and inflammation to Alzheimer’s pathology. Neurobiol. Aging 85, 96–103 (2020).
Popp, J. et al. Markers of neuroinflammation associated with Alzheimer’s disease pathology in older adults. Brain Behav. Immun. 62, 203–211 (2017).
Bettcher, B. M. et al. Cerebrospinal fluid and plasma levels of inflammation differentially relate to CNS markers of Alzheimer’s disease pathology and neuronal damage. J. Alzheimers Dis. 62, 385–397 (2018).
Jack, C. R. Jr. et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Yang, H. S. et al. Plasma IL-12/IFN-γ axis predicts cognitive trajectories in cognitively unimpaired older adults. Alzheimers Dement https://doi.org/10.1002/alz.12399 (2021).
Thome, A. D. et al. Functional alterations of myeloid cells during the course of Alzheimer’s disease. Mol. Neurodegener. 13, 61 (2018).
Saresella, M. et al. IL-33 and its decoy sST2 in patients with Alzheimer’s disease and mild cognitive impairment. J. Neuroinflammation 17, 174 (2020).
Dong, Y. et al. Neutrophil hyperactivation correlates with Alzheimer’s disease progression. Ann. Neurol. 83, 387–405 (2018).
Linton, P. J. & Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 5, 133–139 (2004).
Pawelec, G. et al. T cells and aging, January 2002 update. Front. Biosci. 7, d1056–d1183 (2002).
Danielson, M. et al. Neuroinflammatory markers associate with cognitive decline after major surgery: findings of an explorative study. Ann. Neurol. 87, 370–382 (2020).
Holmes, C., Cunningham, C., Zotova, E., Culliford, D. & Perry, V. H. Proinflammatory cytokines, sickness behavior, and Alzheimer disease. Neurology 77, 212–218 (2011).
Cunningham, C. Systemic inflammation and delirium: important co-factors in the progression of dementia. Biochem. Soc. Trans. 39, 945–953 (2011).
Iwashyna, T. J., Ely, E. W., Smith, D. M. & Langa, K. M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304, 1787–1794 (2010).
Heneka, M. T. et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405 (2015).
Holmes, C. et al. Systemic infection, interleukin 1β, and cognitive decline in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 74, 788–789 (2003).
Holmes, C. et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 73, 768–774 (2009).
Sipila, P. N. et al. Hospital-treated infectious diseases and the risk of dementia: a large, multicohort, observational study with a replication cohort. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(21)00144-4 (2021).
Rakic, S. et al. Systemic infection modifies the neuroinflammatory response in late stage Alzheimer’s disease. Acta Neuropathol. Commun. 6, 88 (2018).
Westin, K. et al. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLoS ONE 7, e30525 (2012).
Pillai, J. A. et al. Inflammatory pathway analytes predicting rapid cognitive decline in MCI stage of Alzheimer’s disease. Ann. Clin. Transl. Neurol. 7, 1225–1239 (2020).
Ewers, M. et al. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Sci. Transl Med. 11, eaav6221 (2019).
Taipa, R. et al. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging 76, 125–132 (2019).
Sperling, R. A. et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 (2011).
Meyer, P. F. et al. Bi-directional association of cerebrospinal fluid immune markers with stage of Alzheimer’s disease pathogenesis. J. Alzheimers Dis. 63, 577–590 (2018).
Bradburn, S., Murgatroyd, C. & Ray, N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. Ageing Res. Rev. 50, 1–8 (2019).
Hamelin, L. et al. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer’s disease. Brain 141, 1855–1870 (2018).
Whelan, C. D. et al. Multiplex proteomics identifies novel CSF and plasma biomarkers of early Alzheimer’s disease. Acta Neuropathol. Commun. 7, 169 (2019).
Larbi, A. et al. Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J. Alzheimers Dis. 17, 91–103 (2009).
Pellicano, M. et al. Immune profiling of Alzheimer patients. J. Neuroimmunol. 242, 52–59 (2012).
McManus, R. M., Mills, K. H. & Lynch, M. A. T cells–protective or pathogenic in Alzheimer’s disease? J. Neuroimmune Pharmacol. 10, 547–560 (2015).
Saresella, M. et al. Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behav. Immun. 25, 539–547 (2011).
Ciccocioppo, F. et al. The characterization of regulatory T-cell profiles in Alzheimer’s disease and multiple sclerosis. Sci. Rep. 9, 8788 (2019).
Saresella, M. et al. PD1 negative and PD1 positive CD4+ T regulatory cells in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 21, 927–938 (2010).
Le Page, A. et al. Differential phenotypes of myeloid-derived suppressor and T regulatory cells and cytokine levels in amnestic mild cognitive impairment subjects compared to mild Alzheimer diseased patients. Front. Immunol. 8, 783 (2017).
Lueg, G. et al. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer’s disease. Neurobiol. Aging 36, 81–89 (2015).
Gate, D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404 (2020).
Zotova, E. et al. Inflammatory components in human Alzheimer’s disease and after active amyloid-β42 immunization. Brain 136, 2677–2696 (2013).
Merlini, M., Kirabali, T., Kulic, L., Nitsch, R. M. & Ferretti, M. T. Extravascular CD3+ T cells in brains of Alzheimer disease patients correlate with tau but not with amyloid pathology: an immunohistochemical study. Neurodegener. Dis. 18, 49–56 (2018).
Zhuang, Z. Q. et al. Gut microbiota is altered in patients with Alzheimer’s disease. J. Alzheimers Dis. 63, 1337–1346 (2018).
Vogt, N. M. et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7, 13537 (2017).
Cattaneo, A. et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 49, 60–68 (2017).
Wilmanski, T. et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 3, 274–286 (2021).
Walker, K. A., Ficek, B. N. & Westbrook, R. Understanding the role of systemic inflammation in Alzheimer’s disease. ACS Chem. Neurosci. 10, 3340–3342 (2019).
Dantzer, R., Konsman, J. P., Bluthe, R. M. & Kelley, K. W. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton. Neurosci. 85, 60–65 (2000).
Engelhardt, B., Vajkoczy, P. & Weller, R. O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 18, 123–131 (2017).
Wendeln, A. C. et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338 (2018).
Tejera, D. et al. Systemic inflammation impairs microglial Aβ clearance through NLRP3 inflammasome. EMBO J. 38, e101064 (2019).
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011).
Simard, A. R., Soulet, D., Gowing, G., Julien, J. P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49, 489–502 (2006).
El Khoury, J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 13, 432–438 (2007).
Town, T. et al. Blocking TGF-β–Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 14, 681–687 (2008).
Naert, G. & Rivest, S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 31, 6208–6220 (2011).
Varvel, N. H. et al. Replacement of brain-resident myeloid cells does not alter cerebral amyloid-β deposition in mouse models of Alzheimer’s disease. J. Exp. Med. 212, 1803–1809 (2015).
Prokop, S. et al. |Impact of peripheral myeloid cells on amyloid-β pathology in Alzheimer’s disease-like mice. J. Exp. Med. 212, 1811–1818 (2015).
Kelly, R. J. et al. Glial activation in AβPP/PS1 mice is associated with infiltration of IFNγ-producing cells. J. Alzheimers Dis. 37, 63–75 (2013).
Zenaro, E. et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 21, 880–886 (2015).
Minogue, A. M. et al. Age-associated dysregulation of microglial activation is coupled with enhanced blood-brain barrier permeability and pathology in APP/PS1 mice. Neurobiol. Aging 35, 1442–1452 (2014).
Cruz Hernandez, J. C. et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat. Neurosci. 22, 413–420 (2019).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Okroglic, S., Widmann, C. N., Urbach, H., Scheltens, P. & Heneka, M. T. Clinical symptoms and risk factors in cerebral microangiopathy patients. PLoS ONE 8, e53455 (2013).
Semmler, A. et al. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp. Neurol. 204, 733–740 (2007).
Sarlus, H. & Heneka, M. T. Microglia in Alzheimer’s disease. J. Clin. Invest. 127, 3240–3249 (2017).
Heneka, M. T. et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678 (2013).
Tancredi, V. et al. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neurosci. Lett. 146, 176–178 (1992).
Murray, C. A., Clements, M. P. & Lynch, M. A. Interleukin-1 induces lipid peroxidation and membrane changes in rat hippocampus: an age-related study. Gerontology 45, 136–142 (1999).
McAlpine, F. E. et al. Inhibition of soluble TNF signaling in a mouse model of Alzheimer’s disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol. Dis. 34, 163–177 (2009).
MacPherson, K. P. et al. Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiol. Dis. 102, 81–95 (2017).
Hewett, S. J., Csernansky, C. A. & Choi, D. W. Selective potentiation of NMDA-induced neuronal injury following induction of astrocytic iNOS. Neuron 13, 487–494 (1994).
Franklin, B. S. et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat. Immunol. 15, 727–737 (2014).
Venegas, C. et al. Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552, 355–361 (2017).
Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 (2018).
Ising, C. et al. NLRP3 inflammasome activation drives tau pathology. Nature 575, 669–673 (2019).
Tejera, D. & Heneka, M. T. In vivo phagocytosis analysis of amyloid beta. Methods Mol. Biol. 2034, 287–292 (2019).
Marsh, S. E. et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc. Natl Acad. Sci. USA 113, E1316–E1325 (2016).
Spani, C. et al. Reduced β-amyloid pathology in an APP transgenic mouse model of Alzheimer’s disease lacking functional B and T cells. Acta Neuropathol. Commun. 3, 71 (2015).
Togo, T. et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 124, 83–92 (2002).
Itagaki, S., McGeer, P. L. & Akiyama, H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer’s disease brain tissue. Neurosci. Lett. 91, 259–264 (1988).
Rogers, J., Luber-Narod, J., Styren, S. D. & Civin, W. H. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging 9, 339–349 (1988).
Rosset, M. B., Lui, G., Dansokho, C., Chaigneau, T. & Dorothee, G. Vaccine-induced Aβ-specific CD8+ T cells do not trigger autoimmune neuroinflammation in a murine model of Alzheimer’s disease. J. Neuroinflammation 12, 95 (2015).
Monsonego, A. et al. Aβ-induced meningoencephalitis is IFN-γ-dependent and is associated with T cell-dependent clearance of Aβ in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 103, 5048–5053 (2006).
Browne, T. C. et al. IFN-γ production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer’s disease. J. Immunol. 190, 2241–2251 (2013).
Cao, C. et al. Aβ-specific Th2 cells provide cognitive and pathological benefits to Alzheimer’s mice without infiltrating the CNS. Neurobiol. Dis. 34, 63–70 (2009).
Ethell, D. W. et al. Aβ-specific T-cells reverse cognitive decline and synaptic loss in Alzheimer’s mice. Neurobiol. Dis. 23, 351–361 (2006).
Toly-Ndour, C. et al. MHC-independent genetic factors control the magnitude of CD4+ T cell responses to amyloid-β peptide in mice through regulatory T cell-mediated inhibition. J. Immunol. 187, 4492–4500 (2011).
Dansokho, C. et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 139, 1237–1251 (2016).
Baruch, K. et al. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun. 6, 7967 (2015).
Laurent, C. et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain 140, 184–200 (2017).
Harach, T. et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 7, 41802 (2017).
Wang, X. et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 29, 787–803 (2019).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Minter, M. R. et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 6, 30028 (2016).
Dodiya, H. B. et al. Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes. J. Exp. Med. 216, 1542–1560 (2019).
Farfara, D. et al. γ-Secretase component presenilin is important for microglia β-amyloid clearance. Ann. Neurol. 69, 170–180 (2011).
Ledo, J. H. et al. Presenilin 1 phosphorylation regulates amyloid-β degradation by microglia. Mol. Psychiatry https://doi.org/10.1038/s41380-020-0856-8 (2020).
Fung, S. et al. Early-onset familial Alzheimer disease variant PSEN2 N141I heterozygosity is associated with altered microglia phenotype. J. Alzheimers Dis. 77, 675–688 (2020).
Glymour, M. M. & Bibbins-Domingo, K. The future of observational epidemiology: improving data and design to align with population health. Am. J. Epidemiol. 188, 836–839 (2019).
Heneka, M. T., Golenbock, D. T. & Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 16, 229–236 (2015).
Morris, J. K., Honea, R. A., Vidoni, E. D., Swerdlow, R. H. & Burns, J. M. Is Alzheimer’s disease a systemic disease? Biochim. Biophys. Acta 1842, 1340–1349 (2014).
Wang, J., Gu, B. J., Masters, C. L. & Wang, Y. J. A systemic view of Alzheimer disease - insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 13, 612–623 (2017).
Ritchie, K., Ritchie, C. W., Yaffe, K., Skoog, I. & Scarmeas, N. Is late-onset Alzheimer’s disease really a disease of midlife? Alzheimers Dement. 1, 122–130 (2015).
Gui, Y., Marks, J. D., Das, S., Hyman, B. T. & Serrano-Pozo, A. Characterization of the 18 kDa translocator protein (TSPO) expression in post-mortem normal and Alzheimer’s disease brains. Brain Pathol. 30, 151–164 (2020).
Boche, D., Gerhard, A., Rodriguez-Vieitez, E. & Faculty, M. Prospects and challenges of imaging neuroinflammation beyond TSPO in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 46, 2831–2847 (2019).
Turkheimer, F. E. et al. Increased serum peripheral C-reactive protein is associated with reduced brain barriers permeability of TSPO radioligands in healthy volunteers and depressed patients: implications for inflammation and depression. Brain Behav. Immun. 91, 487–497 (2021).
Gottesman, R. F. et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 317, 1443–1450 (2017).
Da Mesquita, S., Fu, Z. & Kipnis, J. The meningeal lymphatic system: a new player in neurophysiology. Neuron 100, 375–388 (2018).
Liddelow, S. A. & Barres, B. A. Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 (2017).
McAlpine, C. S. et al. Astrocytic interleukin-3 programs microglia and limits Alzheimer’s disease. Nature 595, 701–706 (2021).
Wildsmith, K. R. et al. In vivo human apolipoprotein E isoform fractional turnover rates in the CNS. PLoS ONE 7, e38013 (2012).
Cenini, G. et al. Dissecting Alzheimer’s disease pathogenesis in human 2D and 3D models. Mol. Cell Neurosci. 110, 103568 (2021).
Shi, Y. & Holtzman, D. M. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 18, 759–772 (2018).
Scheiblich, H., Trombly, M., Ramirez, A. & Heneka, M. T. Neuroimmune connections in aging and neurodegenerative diseases. Trends Immunol. 41, 300–312 (2020).
McGeer, P. L., Rogers, J. & McGeer, E. G. Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. J. Alzheimers Dis. 9, 271–276 (2006).
Arvanitakis, Z. et al. Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology 70, 2219–2225 (2008).
Jansen, I. E. et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 51, 404–413 (2019).
Acknowledgements
This manuscript was facilitated by the Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART), through the Immunity and Neurodegeneration Professional Interest Area (PIA). The views and opinions expressed by the authors in this publication represent those of the authors and do not necessarily reflect those of the PIA membership, ISTAART or the Alzheimer’s Association. B.M.B. is a funded investigator of the NIH/NIA (R01 AG058772) and the Department of Defense CDMRP. M.G.T. is a funded investigator of the NIH/NIA (7RF1AG057247), the Parkinson’s Foundation and the Michael J. Fox Foundation for Parkinson’s Research. G.D. is a funded investigator of the Fondation Plan Alzheimer, Fondation pour la Recherche Medicale, LECMA/Fondation Vaincre Alzheimer and Alzheimer Research UK. M.T.H. is a funded investigator of the German Research Council (DFG) Cluster of Excellence and has received support from this work through an NIH grant (R01 AG059752-02).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.G.T. is a member of the Medical Scientific Advisory Group to the Alzheimer’s Association and a consultant to INmune Bio. G.D. is listed as an inventor on patent WO2014206899A1 related to a peripheral immunomodulatory approach for treating Alzheimer disease and related disorders, and on patent WO2018172540A1 related to peripheral immune biomarkers for predicting the progression of Alzheimer disease. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks O. Garaschuk and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bettcher, B.M., Tansey, M.G., Dorothée, G. et al. Peripheral and central immune system crosstalk in Alzheimer disease — a research prospectus. Nat Rev Neurol 17, 689–701 (2021). https://doi.org/10.1038/s41582-021-00549-x
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41582-021-00549-x
This article is cited by
-
Immune modulation to treat Alzheimer’s disease
Molecular Neurodegeneration (2025)
-
Hampered AMPK-ULK1 cascade in Alzheimer’s disease (AD) instigates mitochondria dysfunctions and AD-related alterations which are alleviated by metformin
Alzheimer's Research & Therapy (2025)
-
Analysis and interpretation of inflammatory fluid markers in Alzheimer’s disease: a roadmap for standardization
Journal of Neuroinflammation (2025)
-
Transcriptomic predictors of rapid progression from mild cognitive impairment to Alzheimer's disease
Alzheimer's Research & Therapy (2025)
-
ASO-mediated knock-down of GPNMB in mutant-GRN and in Grn-deficient peripheral myeloid cells disrupts lysosomal function and immune responses
Molecular Neurodegeneration (2025)