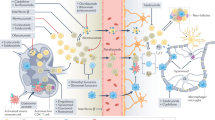
Abstract
Current therapies for multiple sclerosis (MS) reduce both relapses and relapse-associated worsening of disability, which is assumed to be mainly associated with transient infiltration of peripheral immune cells into the central nervous system (CNS). However, approved therapies are less effective at slowing disability accumulation in patients with MS, in part owing to their lack of relevant effects on CNS-compartmentalized inflammation, which has been proposed to drive disability. Bruton tyrosine kinase (BTK) is an intracellular signalling molecule involved in the regulation of maturation, survival, migration and activation of B cells and microglia. As CNS-compartmentalized B cells and microglia are considered central to the immunopathogenesis of progressive MS, treatment with CNS-penetrant BTK inhibitors might curtail disease progression by targeting immune cells on both sides of the blood–brain barrier. Five BTK inhibitors that differ in selectivity, strength of inhibition, binding mechanisms and ability to modulate immune cells within the CNS are currently under investigation in clinical trials as a treatment for MS. This Review describes the role of BTK in various immune cells implicated in MS, provides an overview of preclinical data on BTK inhibitors and discusses the (largely preliminary) data from clinical trials.
Key points
-
Bruton tyrosine kinase (BTK) is an important intracellular signalling molecule involved in regulating the maturation, proliferation, survival and activation of B cells and myeloid cells.
-
BTK inhibitors might concurrently target adaptive and innate immune mechanisms in the periphery and central nervous system (CNS).
-
This ability makes BTK inhibitors a promising therapeutic approach for relapsing and progressive forms of multiple sclerosis (MS).
-
Preclinical studies show that BTK inhibition can suppress key pathological features of MS, including B cell activation, CNS lymphocyte infiltration, leptomeningeal inflammation, pro-inflammatory microglial activation and demyelination.
-
The efficacy and safety of five BTK inhibitors are currently being evaluated in clinical trials in patients with relapsing and progressive MS.
-
The BTK inhibitors under investigation in clinical trials differ in their selectivity, mode of binding, target occupancy, inhibitory potency and CNS penetrance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Reich, D. S., Lucchinetti, C. F. & Calabresi, P. A. Multiple sclerosis. N. Engl. J. Med. 378, 169–180 (2018).
Faissner, S., Plemel, J. R., Gold, R. & Yong, V. W. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 18, 905–922 (2019).
Dendrou, C. A., Fugger, L. & Friese, M. A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015).
Krämer, J. et al. Imaging in mice and men: pathophysiological insights into multiple sclerosis from conventional and advanced MRI techniques. Prog. Neurobiol. 182, 101663 (2019).
Baecher-Allan, C., Kaskow, B. J. & Weiner, H. L. Multiple sclerosis: mechanisms and immunotherapy. Neuron 97, 742–768 (2018).
Bar-Or, A. & Li, R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol. 20, 470–483 (2021).
Healy, L. M., Stratton, J. A., Kuhlmann, T. & Antel, J. The role of glial cells in multiple sclerosis disease progression. Nat. Rev. Neurol. 18, 237–248 (2022).
Ruiz, F., Vigne, S. & Pot, C. Resolution of inflammation during multiple sclerosis. Semin. Immunopathol. 41, 711–726 (2019).
DiSabato, D. J., Quan, N. & Godbout, J. P. Neuroinflammation: the devil is in the details. J. Neurochem. 139, 136–153 (2016).
Minagar, A., Maghzi, A. H., McGee, J. C. & Alexander, J. S. Emerging roles of endothelial cells in multiple sclerosis pathophysiology and therapy. Neurol. Res. 34, 738–745 (2012).
Yong, H. Y. F. & Yong, V. W. Mechanism-based criteria to improve therapeutic outcomes in progressive multiple sclerosis. Nat. Rev. Neurol. 18, 40–55 (2022).
Dangond, F. et al. Facing the urgency of therapies for progressive MS — a progressive MS alliance proposal. Nat. Rev. Neurol. 17, 185–192 (2021).
Lassmann, H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front. Immunol. 9, 3116 (2018).
Magliozzi, R. et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130, 1089–1104 (2007).
Choi, S. R. et al. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 135, 2925–2937 (2012).
Absinta, M. et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J. Clin. Invest. 126, 2597–2609 (2016).
Kuhlmann, T. et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24 (2017).
Lassmann, H. The contribution of neuropathology to multiple sclerosis research. Eur. J. Neurol. 29, 2869–2877 (2022).
Lassmann, H., Raine, C. S., Antel, J. & Prineas, J. W. Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J. Neuroimmunol. 86, 213–217 (1998).
Calvi, A. et al. In vivo imaging of chronic active lesions in multiple sclerosis. Mult. Scler. 28, 683–690 (2022).
Dal-Bianco, A. et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 133, 25–42 (2017).
Absinta, M. et al. A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714 (2021).
Jackle, K. et al. Molecular signature of slowly expanding lesions in progressive multiple sclerosis. Brain 143, 2073–2088 (2020).
Elliott, C. et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult. Scler. 25, 1915–1925 (2019).
Preziosa, P. et al. Slowly expanding lesions predict 9-year multiple sclerosis disease progression. Neurol. Neuroimmunol. Neuroinflamm. 9, e1139 (2022).
Luchetti, S. et al. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 135, 511–528 (2018).
Frischer, J. M. et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 78, 710–721 (2015).
Absinta, M. & Dal-Bianco, A. Slowly expanding lesions are a marker of progressive MS — yes. Mult. Scler. 27, 1679–1681 (2021).
Simmons, S. B. & Ontaneda, D. Slowly expanding lesions: a new target for progressive multiple sclerosis trials. Neurology 98, 699–700 (2022).
Guerrero, B. L. & Sicotte, N. L. Microglia in multiple sclerosis: friend or foe? Front. Immunol. 11, 374 (2020).
Hauser, S. L. et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 376, 221–234 (2017).
Montalban, X. et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 376, 209–220 (2017).
Hauser, S. L. et al. Ofatumumab versus teriflunomide in multiple sclerosis. N. Engl. J. Med. 383, 546–557 (2020).
Hauser, S. L. et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 358, 676–688 (2008).
Hawker, K. et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 66, 460–471 (2009).
Gelfand, J. M., Cree, B. A. C. & Hauser, S. L. Ocrelizumab and other CD20(+) B-cell-depleting therapies in multiple sclerosis. Neurotherapeutics 14, 835–841 (2017).
Bhargava, P. et al. Trial of intrathecal rituximab in progressive multiple sclerosis patients with evidence of leptomeningeal contrast enhancement. Mult. Scler. Relat. Disord. 30, 136–140 (2019).
Komori, M. et al. Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann. Clin. Transl. Neurol. 3, 166–179 (2016).
Li, R., Patterson, K. R. & Bar-Or, A. Reassessing B cell contributions in multiple sclerosis. Nat. Immunol. 19, 696–707 (2018).
Bonnan, M. et al. No early effect of intrathecal rituximab in progressive multiple sclerosis (EFFRITE Clinical Trial). Mult. Scler. Int. 2021, 8813498–707 (2021).
Torke, S. & Weber, M. S. Inhibition of Bruton’s tyrosine kinase as a novel therapeutic approach in multiple sclerosis. Exp. Opin. Investig. Drugs 29, 1143–1150 (2020).
Brunner, C., Müller, B. & Wirth, T. Bruton’s tyrosine kinase is involved in innate and adaptive immunity. Histol. Histopathol. 20, 945–955 (2005).
Rip, J., Van Der Ploeg, E. K., Hendriks, R. W. & Corneth, O. B. J. The role of Bruton’s tyrosine kinase in immune cell signaling and systemic autoimmunity. Crit. Rev. Immunol. 38, 17–62 (2018).
Torke, S. et al. Inhibition of Bruton’s tyrosine kinase interferes with pathogenic B-cell development in inflammatory CNS demyelinating disease. Acta Neuropathol. 140, 535–548 (2020).
Carnero Contentti, E. & Correale, J. Bruton’s tyrosine kinase inhibitors: a promising emerging treatment option for multiple sclerosis. Exp. Opin. Emerg. Drugs 25, 377–381 (2020).
Smith, C. I. et al. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J. Immunol. 152, 557–565 (1994).
Garcia-Merino, A. Bruton’s tyrosine kinase inhibitors: a new generation of promising agents for multiple sclerosis therapy. Cells 10, 2560 (2021).
Ellmeier, W., Abramova, A. & Schebesta, A. Tec family kinases: regulation of FcεRI-mediated mast-cell activation. FEBS J. 278, 1990–2000 (2011).
Hata, D. et al. Involvement of Bruton’s tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J. Exp. Med. 187, 1235–1247 (1998).
Iwaki, S. et al. Btk plays a crucial role in the amplification of FcεRI-mediated mast cell activation by kit. J. Biol. Chem. 280, 40261–40270 (2005).
Yang, E. J., Yoon, J. H. & Chung, K. C. Bruton’s tyrosine kinase phosphorylates cAMP-responsive element-binding protein at serine 133 during neuronal differentiation in immortalized hippocampal progenitor cells. J. Biol. Chem. 279, 1827–1837 (2004).
Martin, E. et al. Bruton’s tyrosine kinase inhibition promotes myelin repair. Brain Plast. 5, 123–133 (2020).
Yu, C. G. et al. Inhibition of Bruton tyrosine kinase reduces neuroimmune cascade and promotes recovery after spinal cord injury. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23010355 (2021).
Bruton, O. C. Agammaglobulinemia. Pediatrics 9, 722–728 (1952).
Vetrie, D. et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature 361, 226–233 (1993).
Tsukada, S. et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell 72, 279–290 (1993).
Hendriks, R. W., Yuvaraj, S. & Kil, L. P. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat. Rev. Cancer 14, 219–232 (2014).
Mahajan, S. et al. Rational design and synthesis of a novel anti-leukemic agent targeting Bruton’s tyrosine kinase (BTK), LFM-A13 [alpha-cyano-beta-hydroxy-beta-methyl-N-(2,5-dibromophenyl)propenamide]. J. Biol. Chem. 274, 9587–9599 (1999).
Kiss, R. et al. Identification of a novel inhibitor of JAK2 tyrosine kinase by structure-based virtual screening. Bioorg. Med. Chem. Lett. 19, 3598–3601 (2009).
van den Akker, E. et al. The Btk inhibitor LFM-A13 is a potent inhibitor of Jak2 kinase activity. Biol. Chem. 385, 409–413 (2004).
Pan, Z. et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem 2, 58–61 (2007).
Wen, T., Wang, J., Shi, Y., Qian, H. & Liu, P. Inhibitors targeting Bruton’s tyrosine kinase in cancers: drug development advances. Leukemia 35, 312–332 (2021).
Jansset Biotech, Inc. Imbruvica (Ibrutinib) Capsules and Tablets [Prescribing Information] (Jansset Biotech, Inc., 2020).
AstraZeneca. Calquence (Acalabrutinib) Capsules [Prescribing Information] (AstraZeneca, 2019).
BeiGene USA, Inc. Brukinsa (Zanubrutinib) Capsules [Prescribing Information] (BeiGene USA, Inc., 2019).
Estupinan, H. Y., Berglof, A., Zain, R. & Smith, C. I. E. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Front. Cell Dev. Biol. 9, 630942 (2021).
Ringheim, G. E., Wampole, M. & Oberoi, K. Bruton’s tyrosine kinase (BTK) inhibitors and autoimmune diseases: making sense of BTK inhibitor specificity profiles and recent clinical trial successes and failures. Front. Immunol. 12, 662223 (2021).
Corneth, O. B. et al. Enhanced expression of Bruton’s tyrosine kinase in B cells drives systemic autoimmunity by disrupting T cell homeostasis. J. Immunol. 197, 58–67 (2016).
Kil, L. P. et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood 119, 3744–3756 (2012).
Mano, H. Tec family of protein-tyrosine kinases: an overview of their structure and function. Cytokine Growth Factor Rev. 10, 267–280 (1999).
Dickson, E. J. & Hille, B. Understanding phosphoinositides: rare, dynamic, and essential membrane phospholipids. Biochem. J. 476, 1–23 (2019).
Solvason, N. et al. Transgene expression of bcl-xL permits anti-immunoglobulin (Ig)-induced proliferation in xid B cells. J. Exp. Med. 187, 1081–1091 (1998).
Uckun, F. M. et al. BTK as a mediator of radiation-induced apoptosis in DT-40 lymphoma B cells. Science 273, 1096–1100 (1996).
Smith, C. I. et al. The Tec family of cytoplasmic tyrosine kinases: mammalian Btk, Bmx, Itk, Tec, Txk and homologs in other species. Bioessays 23, 436–446 (2001).
Ekman, N. et al. The BMX tyrosine kinase is activated by IL-3 and G-CSF in a PI-3K dependent manner. Oncogene 19, 4151–4158 (2000).
Schaeffer, E. M. et al. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science 284, 638–641 (1999).
Basile, N. et al. Clinical and molecular analysis of 49 patients with X-linked agammaglobulinemia from a single center in Argentina. J. Clin. Immunol. 29, 123–129 (2009).
Väliaho, J., Smith, C. I. & Vihinen, M. BTKbase: the mutation database for X-linked agammaglobulinemia. Hum. Mutat. 27, 1209–1217 (2006).
Rawlings, D. J. et al. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science 261, 358–361 (1993).
Cancro, M. P. et al. xid mice reveal the interplay of homeostasis and Bruton’s tyrosine kinase-mediated selection at multiple stages of B cell development. Int. Immunol. 13, 1501–1514 (2001).
Thomas, J. D. et al. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science 261, 355–358 (1993).
Middendorp, S., Dingjan, G. M. & Hendriks, R. W. Impaired precursor B cell differentiation in Bruton’s tyrosine kinase-deficient mice. J. Immunol. 168, 2695–2703 (2002).
Ng, Y. S., Wardemann, H., Chelnis, J., Cunningham-Rundles, C. & Meffre, E. Bruton’s tyrosine kinase is essential for human B cell tolerance. J. Exp. Med. 200, 927–934 (2004).
Cariappa, A. et al. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, BTK, and CD21. Immunity 14, 603–615 (2001).
Spaargaren, M. et al. The B cell antigen receptor controls integrin activity through Btk and PLCgamma2. J. Exp. Med. 198, 1539–1550 (2003).
Lunemann, J. D., Malhotra, S., Shinohara, M. L., Montalban, X. & Comabella, M. Targeting inflammasomes to treat neurological diseases. Ann. Neurol. 90, 177–188 (2021).
Schmitz, R., Baumann, G. & Gram, H. Catalytic specificity of phosphotyrosine kinases Blk, Lyn, c-Src and Syk as assessed by phage display. J. Mol. Biol. 260, 664–677 (1996).
Fujimoto, M. et al. CD19 regulates Src family protein tyrosine kinase activation in B lymphocytes through processive amplification. Immunity 13, 47–57 (2000).
Fütterer, K., Wong, J., Grucza, R. A., Chan, A. C. & Waksman, G. Structural basis for Syk tyrosine kinase ubiquity in signal transduction pathways revealed by the crystal structure of its regulatory SH2 domains bound to a dually phosphorylated ITAM peptide. J. Mol. Biol. 281, 523–537 (1998).
Rowley, R. B., Burkhardt, A. L., Chao, H. G., Matsueda, G. R. & Bolen, J. B. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Ig alpha/Ig beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J. Biol. Chem. 270, 11590–11594 (1995).
Xu, Y., Fairfax, K., Light, A., Huntington, N. D. & Tarlinton, D. M. CD19 differentially regulates BCR signalling through the recruitment of PI3K. Autoimmunity 47, 430–437 (2014).
Okada, T., Maeda, A., Iwamatsu, A., Gotoh, K. & Kurosaki, T. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity 13, 817–827 (2000).
Saito, K., Scharenberg, A. M. & Kinet, J. P. Interaction between the Btk PH domain and phosphatidylinositol-3,4,5-trisphosphate directly regulates BTK. J. Biol. Chem. 276, 16201–16206 (2001).
Haxhinasto, S. A. & Bishop, G. A. Synergistic B cell activation by CD40 and the B cell antigen receptor: role of B lymphocyte antigen receptor-mediated kinase activation and tumor necrosis factor receptor-associated factor regulation. J. Biol. Chem. 279, 2575–2582 (2004).
Ying, H., Li, Z., Yang, L. & Zhang, J. Syk mediates BCR- and CD40-signaling integration during B cell activation. Immunobiology 216, 566–570 (2011).
Tanwar, S. et al. Mediation of transitional B cell maturation in the absence of functional Bruton’s tyrosine kinase. Sci. Rep. 7, 46029 (2017).
Su, Y. W. et al. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur. J. Immunol. 29, 3702–3711 (1999).
Liu, W. S. & Heckman, C. A. The sevenfold way of PKC regulation. Cell. Signal. 10, 529–542 (1998).
Taylor, C. W. & Tovey, S. C. IP(3) receptors: toward understanding their activation. Cold Spring Harb. Perspect. Biol. 2, a004010 (2010).
Lin, X. & Wang, D. The roles of CARMA1, Bcl10, and MALT1 in antigen receptor signaling. Semin. Immunol. 16, 429–435 (2004).
David, L. et al. Assembly mechanism of the CARMA1-BCL10-MALT1-TRAF6 signalosome. Proc. Natl Acad. Sci. USA 115, 1499–1504 (2018).
Shinohara, H., Maeda, S., Watarai, H. & Kurosaki, T. IkappaB kinase beta-induced phosphorylation of CARMA1 contributes to CARMA1 Bcl10 MALT1 complex formation in B cells. J. Exp. Med. 204, 3285–3293 (2007).
Mohamed, A. J. et al. Bruton’s tyrosine kinase (BTK): function, regulation, and transformation with special emphasis on the PH domain. Immunol. Rev. 228, 58–73 (2009).
Berland, R. & Wortis, H. H. Normal B-1a cell development requires B cell-intrinsic NFATc1 activity. Proc. Natl Acad. Sci. USA 100, 13459–13464 (2003).
Peng, S. L., Gerth, A. J., Ranger, A. M. & Glimcher, L. H. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity 14, 13–20 (2001).
Bhattacharyya, S. et al. NFATc1 affects mouse splenic B cell function by controlling the calcineurin–NFAT signaling network. J. Exp. Med. 208, 823–839 (2011).
Barrington, R. A., Borde, M., Rao, A. & Carroll, M. C. Involvement of NFAT1 in B cell self-tolerance. J. Immunol. 177, 1510–1515 (2006).
Gerondakis, S. & Siebenlist, U. Roles of the NF-kappaB pathway in lymphocyte development and function. Cold Spring Harb. Perspect. Biol. 2, a000182 (2010).
Teixeira, C., Stang, S. L., Zheng, Y., Beswick, N. S. & Stone, J. C. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood 102, 1414–1420 (2003).
Yasuda, T. et al. Erk kinases link pre-B cell receptor signaling to transcriptional events required for early B cell expansion. Immunity 28, 499–508 (2008).
Westerberg, L., Greicius, G., Snapper, S. B., Aspenström, P. & Severinson, E. Cdc42, Rac1, and the Wiskott–Aldrich syndrome protein are involved in the cytoskeletal regulation of B lymphocytes. Blood 98, 1086–1094 (2001).
O’Rourke, L. M. et al. CD19 as a membrane-anchored adaptor protein of B lymphocytes: costimulation of lipid and protein kinases by recruitment of Vav. Immunity 8, 635–645 (1998).
Eden, S., Rohatgi, R., Podtelejnikov, A. V., Mann, M. & Kirschner, M. W. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 418, 790–793 (2002).
Chiu, C. W., Dalton, M., Ishiai, M., Kurosaki, T. & Chan, A. C. BLNK: molecular scaffolding through ‘cis’-mediated organization of signaling proteins. EMBO J. 21, 6461–6472 (2002).
Muta, T. et al. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature 368, 70–73 (1994).
Nishizumi, H., Horikawa, K., Mlinaric-Rascan, I. & Yamamoto, T. A double-edged kinase Lyn: a positive and negative regulator for antigen receptor-mediated signals. J. Exp. Med. 187, 1343–1348 (1998).
Jefferies, C. A. et al. Bruton’s tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J. Biol. Chem. 278, 26258–26264 (2003).
Gray, P. et al. MyD88 adapter-like (Mal) is phosphorylated by Bruton’s tyrosine kinase during TLR2 and TLR4 signal transduction. J. Biol. Chem. 281, 10489–10495 (2006).
Lee, K. G. et al. Bruton’s tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proc. Natl Acad. Sci. USA 109, 5791–5796 (2012).
O’Neill, L. A. & Bowie, A. G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7, 353–364 (2007).
Botos, I., Segal, D. M. & Davies, D. R. The structural biology of Toll-like receptors. Structure 19, 447–459 (2011).
Muroi, M. & Tanamoto, K. TRAF6 distinctively mediates MyD88- and IRAK-1-induced activation of NF-kappaB. J. Leukoc. Biol. 83, 702–707 (2008).
Rip, J. et al. Toll-like receptor signaling drives BTK-mediated autoimmune disease. Front. Immunol. 10, 95 (2019).
Kenny, E. F. et al. Bruton’s tyrosine kinase mediates the synergistic signalling between TLR9 and the B cell receptor by regulating calcium and calmodulin. PLoS ONE 8, e74103 (2013).
Kawakami, Y. et al. Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon Fc epsilon RI cross-linking. Mol. Cell Biol. 14, 5108–5113 (1994).
Furumoto, Y., Nunomura, S., Terada, T., Rivera, J. & Ra, C. The FcϵRIβ immunoreceptor tyrosine-based activation motif exerts inhibitory control on MAPK and IκB kinase phosphorylation and mast cell cytokine production. J. Biol. Chem. 279, 49177–49187 (2004).
Simon, M., Vanes, L., Geahlen, R. L. & Tybulewicz, V. L. Distinct roles for the linker region tyrosines of Syk in FcepsilonRI signaling in primary mast cells. J. Biol. Chem. 280, 4510–4517 (2005).
Saitoh, S. et al. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity 12, 525–535 (2000).
Gilfillan, A. M. & Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 6, 218–230 (2006).
Jongstra-Bilen, J. et al. Dual functions of Bruton’s tyrosine kinase and Tec kinase during Fcgamma receptor-induced signaling and phagocytosis. J. Immunol. 181, 288–298 (2008).
Liang, C. et al. The development of Bruton’s tyrosine kinase (BTK) inhibitors from 2012 to 2017: a mini-review. Eur. J. Med. Chem. 151, 315–326 (2018).
Pal Singh, S., Dammeijer, F. & Hendriks, R. W. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer 17, 57 (2018).
Di Paolo, J. A. et al. Specific BTK inhibition suppresses B cell- and myeloid cell-mediated arthritis. Nat. Chem. Biol. 7, 41–50 (2011).
Kiefer, F. et al. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol. Cell Biol. 18, 4209–4220 (1998).
Horwood, N. J. et al. Bruton’s tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. J. Immunol. 176, 3635–3641 (2006).
Doyle, S. L., Jefferies, C. A., Feighery, C. & O’Neill, L. A. Signaling by Toll-like receptors 8 and 9 requires Bruton’s tyrosine kinase. J. Biol. Chem. 282, 36953–36960 (2007).
Liu, X. et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase BTK. Nat. Immunol. 12, 416–424 (2011).
Rijvers, L. et al. Human T-bet + B cell development is associated with BTK activity and suppressed by evobrutinib. JCI Insight https://doi.org/10.1172/jci.insight.160909 (2022).
Gruber, R. et al. Central effects of BTK inhibition in neuroinflammation. Neurology 94, 808 (2020).
Gruber, R. et al. Evaluating the effect of BTK inhibitor tolebrutinib in human microglia. Mult. Scler. 27, P391 (2021).
Cui, L. Y., Chu, S. F. & Chen, N. H. The role of chemokines and chemokine receptors in multiple sclerosis. Int. Immunopharmacol. 83, 106314 (2020).
Kowarik, M. C. et al. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J. Neuroinflammation 9, 93 (2012).
Ransohoff, R. M. & Perry, V. H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 (2009).
Luo, C. et al. The role of microglia in multiple sclerosis. Neuropsychiatr. Dis. Treat. 13, 1661–1667 (2017).
Dong, Y. & Yong, V. W. When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat. Rev. Neurol. 15, 704–717 (2019).
Kamma, E., Lasisi, W., Libner, C., Ng, H. S. & Plemel, J. R. Central nervous system macrophages in progressive multiple sclerosis: relationship to neurodegeneration and therapeutics. J. Neuroinflammation 19, 45 (2022).
Puthenparampil, M. et al. BAFF index and CXCL13 levels in the cerebrospinal fluid associate respectively with intrathecal IgG synthesis and cortical atrophy in multiple sclerosis at clinical onset. J. Neuroinflammation 14, 11 (2017).
Jain, R. W. & Yong, V. W. B cells in central nervous system disease: diversity, locations and pathophysiology. Nat. Rev. Immunol. 22, 513–524 (2021).
Arneth, B. M. Impact of B cells to the pathophysiology of multiple sclerosis. J. Neuroinflammation 16, 128 (2019).
Flach, A. C. et al. Autoantibody-boosted T-cell reactivation in the target organ triggers manifestation of autoimmune CNS disease. Proc. Natl Acad. Sci. USA 113, 3323–3328 (2016).
Owens, T. D. et al. Phase 1 clinical trial evaluating safety, exposure and pharmacodynamics of BTK inhibitor tolebrutinib (PRN2246, SAR442168). Clin. Transl. Sci. 15, 442–450 (2022).
Caldwell, R. D. et al. Discovery of evobrutinib: an oral, potent, and highly selective, covalent Bruton’s tyrosine kinase (BTK) inhibitor for the treatment of immunological diseases. J. Med. Chem. 62, 7643–7655 (2019).
Comi, G. et al. Role of B cells in multiple sclerosis and related disorders. Ann. Neurol. 89, 13–23 (2021).
Cencioni, M. T., Mattoscio, M., Magliozzi, R., Bar-Or, A. & Muraro, P. A. B cells in multiple sclerosis — from targeted depletion to immune reconstitution therapies. Nat. Rev. Neurol. 17, 399–414 (2021).
Nyhoff, L. E. et al. Bruton’s tyrosine kinase is not essential for B cell survival beyond early developmental stages. J. Immunol. 200, 2352–2361 (2018).
Bame, E. et al. Next-generation Bruton’s tyrosine kinase inhibitor BIIB091 selectively and potently inhibits B cell and Fc receptor signaling and downstream functions in B cells and myeloid cells. Clin. Transl. Immunol. 10, e1295 (2021).
de Rooij, M. F. et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 119, 2590–2594 (2012).
Herman, S. E. et al. Ibrutinib-induced lymphocytosis in patients with chronic lymphocytic leukemia: correlative analyses from a phase II study. Leukemia 28, 2188–2196 (2014).
Francesco, M. et al. PRN2246, a potent and selective blood–brain barrier penetrating BTK inhibitor, exhibits efficacy in central nervous system immunity. Mult. Scler. 23, P989 (2017).
Bassani, C. et al. The role of human and mouse BTK in myeloid cells (P3-4.006). Neurology 98, 2707 (2022).
Boschert, U. et al. T cell mediated experimental CNS autoimmunity induced by PLP in SJL mice is modulated by evobrutinib (M2951): a novel Bruton’s tyrosine kinase inhibitor. Mult. Scler. 23, P678 (2017).
Bhargava, P. et al. Imaging meningeal inflammation in CNS autoimmunity identifies a therapeutic role for BTK inhibition. Brain 144, 1396–1408 (2021).
Li, R. et al. BTK inhibition limits B-cell–T-cell interaction through modulation of B-cell metabolism: implications for multiple sclerosis therapy. Acta Neuropathol. 143, 505–521 (2022).
Wang, J. et al. Targeting microglia and macrophages: a potential treatment strategy for multiple sclerosis. Front. Pharmacol. 10, 286 (2019).
Mangla, A. et al. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood 104, 1191–1197 (2004).
Alankus, Y., Grenningloh, R., Haselmayer, P., Bender, A. & Bruttger, J. BTK inhibition prevents inflammatory macrophage differentiation: a potential role in MS. Mult. Scler. 24, P557 (2018).
Pellerin, K. et al. MOG autoantibodies trigger a tightly-controlled FcR and BTK-driven microglia proliferative response. Brain 144, 2361–2374 (2021).
Weber, M. et al. Fenebrutinib reduces disease activity in a mouse model of infammatory multiple sclerosis, which is associated with reduced microglial activation. Mult. Scler. 27, P680 (2021).
Gruber, R. et al. Decoding Bruton’s tyrosine kinase signaling in neuroinflammation. Mult. Scler. 26, P0311 (2020).
Gruber, R. et al. Evaluating the effect of a Bruton’s tyrosine kinase inhibitor in a murine experimental autoimmune encephalomyelitis model of multiple sclerosis. Mult. Scler. 28, P174 (2022).
Geladaris, A. et al. Targeting BTK in chronic CNS autoimmunity inhibits activation of microglia. Mult. Scler. 27, P971 (2021).
Barr, H. et al. Microglial BTK signaling regulates immune-mediated cortical demyelination. Mult. Scler. 27, P196 (2021).
Brullo, C., Villa, C., Tasso, B., Russo, E. & Spallarossa, A. BTK inhibitors: a medicinal chemistry and drug delivery perspective. Int. J. Mol. Sci. 22, 7641 (2021).
Zain, R. & Vihinen, M. Structure–function relationships of covalent and non-covalent BTK inhibitors. Front. Immunol. 12, 694853 (2021).
Baillie, T. A. Targeted covalent inhibitors for drug design. Angew. Chem. Int. Ed. Engl. 55, 13408–13421 (2016).
Crawford, J. J. et al. Discovery of GDC-0853: a potent, selective, and noncovalent Bruton’s tyrosine kinase inhibitor in early clinical development. J. Med. Chem. 61, 2227–2245 (2018).
Weber, M. et al. Fenebrutinib demonstrates the highest potency of Bruton tyrosine kinase inhibitors (BTKis) in phase 3 clinical development for multiple sclerosis (MS). Neurology 96, 4437 (2021).
Turner, T., Brun, P., Ofengheim, D. & Gruber, R. Comparative CNS pharmacology of tolebrutinib versus other BTK inhibitor candidates for treating MS. Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum (West Palm Beach, FL, USA) 162 (ACTRIMS, 2022).
Piasecka-Stryczynska, K. et al. Concentration of evobrutinib, a BTK inhibitor, in cerebrospinal fluid during treatment of patients with relapsing multiple sclerosis in a phase 2 study. Mult. Scler. Relat. Disord. 51, 103001 (2021).
Smith, P. et al. Phase 1 clinical trial of PRN2246 (SAR441268), a covalent BTK inhibitor demonstrates safety, CNS exposure and therapeutic levels of BTK occupancy. Mult. Scler. 25, P072 (2019).
Lipsky, A. & Lamanna, N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematol. Am. Soc. Hematol. Educ. Program 2020, 336–345 (2020).
Fiorcari, S. et al. BTK inhibition impairs the innate response against fungal infection in patients with chronic lymphocytic leukemia. Front. Immunol. 11, 2158 (2020).
Tam, C. S. et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood 136, 2038–2050 (2020).
Rotstein, D. L. All Bruton’s tyrosine kinase inhibitors have similar efficacy and risks: no. Mult. Scler. 28, 1500–1502 (2022).
Montalban, X. et al. Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N. Engl. J. Med. 380, 2406–2417 (2019).
Reich, D. S. et al. Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 20, 729–738 (2021).
Vermersch, P. et al. MRI and clinical outcomes of evobrutinib, a Bruton’s tyrosine kinase inhibitor, in relapsing multiple sclerosis over 2.5 years of the open-label extension to a phase 2 trial. Mult. Scler. 28, P731 (2022).
Montalban, X. et al. Safety and efficacy of evobrutinib, a Bruton tyrosine kinase inhibitor, in relapsing multiple sclerosis over 2.5 years of the open-label extension to a phase 2 trial. Int. J. MS Care 21, DMT02 (2022).
Oh, J. et al. MRI, safety, and efficacy outcomes in patients with relapsing MS: 18-month results from the long-term extension study of tolebrutinib. Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum (West Palm Beach, FL, USA) 102 (ACTRIMS, 2022).
Kuhle, J. et al. Evobrutinib, a Bruton’s tyrosine kinase inhibitor, decreases neurofilament light chain levels over 2.5 years of treatment in patients with relapsing multiple sclerosis. Mult. Scler. 28, EP1021 (2022).
Syed, S. et al. Efficacy and safety of tolebrutinib in patients with highly active relapsing MS: subgroup analysis of the phase 2b study. Neurology 96, 2260 (2021).
Lee, D. S. W., Rojas, O. L. & Gommerman, J. L. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat. Rev. Drug Discov. 20, 179–199 (2021).
Correale, J. BTK inhibitors as potential therapies for multiple sclerosis. Lancet Neurol. 20, 689–691 (2021).
Häusser-Kinzel, S. & Weber, M. S. The role of B cells and antibodies in multiple sclerosis, neuromyelitis optica, and related disorders. Front. Immunol. 10, 201 (2019).
Jeske, S. S. et al. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol. Immunother. 69, 1205–1216 (2020).
Lünemann, J. D., Ruck, T., Muraro, P. A., Bar-Or, A. & Wiendl, H. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat. Rev. Neurol. 16, 56–62 (2020).
Ruck, T., Nimmerjahn, F., Wiendl, H. & Lünemann, J. D. Next-generation antibody-based therapies in neurology. Brain 145, 1229–1241 (2022).
Steinmaurer, A., Wimmer, I., Berger, T., Rommer, P. S. & Sellner, J. Bruton’s tyrosine kinase inhibition in the treatment of preclinical models and multiple sclerosis. Curr. Pharm. Des. 28, 437–444 (2022).
Bauer, R. A. Covalent inhibitors in drug discovery: from accidental discoveries to avoided liabilities and designed therapies. Drug Discov. Today 20, 1061–1073 (2015).
Timofeeva, N. & Gandhi, V. Ibrutinib combinations in CLL therapy: scientific rationale and clinical results. Blood Cancer J. 11, 79 (2021).
Tsouki, F. & Williams, A. Multifaceted involvement of microglia in gray matter pathology in multiple sclerosis. Stem Cell 39, 993–1007 (2021).
Sucksdorff, M. et al. Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis. Brain 143, 3318–3330 (2020).
Becker, A. et al. Safety, tolerability, pharmacokinetics, target occupancy, and concentration-QT analysis of the novel BTK inhibitor evobrutinib in healthy volunteers. Clin. Transl. Sci. 13, 325–336 (2020).
Haselmayer, P. et al. Efficacy and pharmacodynamic modeling of the BTK inhibitor evobrutinib in autoimmune disease models. J. Immunol. 202, 2888–2906 (2019).
Herman, A. E. et al. Safety, pharmacokinetics, and pharmacodynamics in healthy volunteers treated with GDC-0853, a selective reversible Bruton’s tyrosine kinase inhibitor. Clin. Pharmacol. Ther. 103, 1020–1028 (2018).
Angst, D. et al. Discovery of LOU064 (remibrutinib), a potent and highly selective covalent inhibitor of Bruton’s tyrosine kinase. J. Med. Chem. 63, 5102–5118 (2020).
Gabizon, R. & London, N. A fast and clean BTK inhibitor. J. Med. Chem. 63, 5100–5101 (2020).
Kaul, M. et al. Remibrutinib (LOU064): a selective potent oral BTK inhibitor with promising clinical safety and pharmacodynamics in a randomized phase I trial. Clin. Transl. Sci. 14, 1756–1768 (2021).
Zhang, B. et al. Orelabrutinib, a potent and selective Bruton’s tyrosine kinase inhibitor with superior safety profile and excellent PK/PD properties. Cancer Res. 80, CT132 (2020).
Nimmerjahn, F. & Ravetch, J. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 (2008).
Bonilla, F. A. & Oettgen, H. C. Adaptive immunity. J. Allergy Clin. Immunol. 125, S33–S40 (2010).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Medzhitov, R. & Janeway, C. Jr. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173, 89–97 (2000).
Li, Q. & Barres, B. A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 18, 225–242 (2018).
Mrdjen, D. et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 599 (2018).
Lloyd, A. F. & Miron, V. E. The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 15, 447–458 (2019).
Baaklini, C. S., Rawji, K. S., Duncan, G. J., Ho, M. F. S. & Plemel, J. R. Central nervous system remyelination: roles of glia and innate immune cells. Front. Mol. Neurosci. 12, 225 (2019).
Acknowledgements
The research of authors is supported by the German Research Council (DFG) grants SFB-CRC TR128 A09 and SFB-CRC TR128 Z02 and funding from Deutsche Myasthenie Gesellschaft, all to H.W. Editorial and graphics assistance was provided by Elevate Scientific Solutions, a division of Envision Pharma Group, and funded by Sanofi. Writing assistance (assistance with drafting of the manuscript text and tables as directed by the authors, data checking and incorporation of comments from reviewers) was provided by R. J. Hogan and C. Underwood of Elevate Scientific Solutions.
Author information
Authors and Affiliations
Contributions
J.K. and H.W. researched data for the article and J.K. wrote the first draft. All authors contributed substantially to discussions of the manuscript content, critically reviewed the drafts and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
J.K. declares that she has received honoraria for lecturing from Biogen, Merck, Mylan, Novartis, Roche, Sanofi and Teva and has received financial research support from Amicus Therapeutics and Sanofi. A.B.-O. declares that he has received grant support to the University of Pennsylvania from Biogen Idec, EMD Serono, Novartis and Roche Genentech. He has participated as a speaker in meetings sponsored by and received consulting fees from Accure, Atara Biotherapeutics, Biogen, Bristol-Myers Squibb, GlaxoSmithKline, Gossamer, Janssen, Medimmune, EMD Serono, Novartis, Roche Genentech and Sanofi. T.J.T. is an employee of and may have ownership interests in Sanofi. H.W. declares that he has acted as a member of the Scientific Advisory Boards of Abbvie, Alexion, Argenx, Bristol Myers Squibb, Janssen, Merck and Novartis. He also declares that he has received speaker’s honoraria and travel support from Alexion, Biogen, Bristol-Myers Squibb, F. Hoffmann-La Roche, Genzyme, Merck, Neurodiem, Novartis, Roche, Teva and WebMD Global and acts as a paid consultant for Abbvie, Actelion, Argenx, Biogen, Bristol-Myers Squibb, EMD Serono, Fondazione Cariplo, Gossamer Bio, Idorsia, Immunic, Immunovant, Janssen, Lundbeck, Merck, NexGen, Novartis, PSI Contract Research Organization, Roche, Sanofi, UCB and Worldwide Clinical Trials. His research is funded by Alexion, Amicus Therapeutics, Argenx, Biogen, CSL Behring, F. Hoffmann-La Roche, Genzyme, Merck, Novartis, Roche and UCB.
Peer review
Peer review information
Nature Reviews Neurology thanks Marco Salvetti, who co-reviewed with Gianmarco Bellucci; Roland Liblau; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria PubMed was searched between 10 November 2021 and 5 May 2022 using terms related to BTK (Bruton’s tyrosine kinase, BTKi*, BTK inhibit*, Bruton’s tyrosine kinase inhibit*) in combination with the following terms: MS OR multiple sclerosis, immune cell*, cancer OR tumour OR tumour OR malignanc*, immune deficienc* OR immune disease* OR immunodeficienc*, mechanism of action OR MOA, B cell OR microglia OR macrophage, pharmacodynamic* OR pharmacokinetic* OR pharmacolog*, safe* OR adverse effect* OR adverse event*. Searches did not use date, language or other filters. All article types were included if the topic was potentially relevant. Results were initially selected according to the article title and then were screened on the basis of the abstract. The full text was retrieved for all potentially relevant articles.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krämer, J., Bar-Or, A., Turner, T.J. et al. Bruton tyrosine kinase inhibitors for multiple sclerosis. Nat Rev Neurol 19, 289–304 (2023). https://doi.org/10.1038/s41582-023-00800-7
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-023-00800-7
This article is cited by
-
A molecular circuit at the brain border that drives gray matter injury
Nature Immunology (2026)
-
In Silico Evaluation of Lipid-Drug Conjugates: Pharmacokinetic and Pharmacodynamic Profiling for Therapeutic Repurposing of Naloxone in Migraine Management
Journal of Pharmaceutical Innovation (2026)
-
Patient-reported gradual worsening reveals progression beyond MS subtypes
Journal of Neurology (2026)
-
Lymphotoxin-dependent elevated meningeal CXCL13:BAFF ratios drive gray matter injury
Nature Immunology (2026)
-
Advancements and challenges in the epidemiology, diagnosis, treatment, and management of multiple sclerosis: a comprehensive review
Beni-Suef University Journal of Basic and Applied Sciences (2025)