Abstract
Diabetic peripheral neuropathy (DPN) is length-dependent peripheral nerve damage arising as a complication of type 1 or type 2 diabetes in up to 50% of patients. DPN poses a substantial burden on patients, who can experience impaired gait and loss of balance, predisposing them to falls and fractures, and neuropathic pain, which is frequently difficult to treat and reduces quality of life. Advanced DPN can lead to diabetic foot ulcers and non-healing wounds that often necessitate lower-limb amputation. From a socioeconomic perspective, DPN increases both direct health-care costs and indirect costs from loss of productivity owing to neuropathy-related disability. In this Review, we highlight the importance of understanding country-specific and region-specific variations in DPN prevalence to inform public health policy and allocate resources appropriately. We also explore how identification of DPN risk factors can guide treatment and prevention strategies and aid the development of health-care infrastructure for populations at risk. We review evidence that metabolic factors beyond hyperglycaemia contribute to DPN development, necessitating a shift from pure glycaemic control to multi-targeted metabolic control, including weight loss and improvements in lipid profiles.
Key points
-
Diabetic peripheral neuropathy (DPN) is length-dependent peripheral nerve damage arising as a complication of type 1 or type 2 diabetes.
-
At the individual level, DPN imposes a substantial clinical burden on patients, including impaired gait and neuropathic pain, as well as diabetic foot ulcers, which can necessitate lower-limb amputation if they become non-healing and infected.
-
From a socioeconomic perspective, patients with DPN incur substantially greater direct and indirect costs than patients with diabetes alone.
-
Determining the prevalence of DPN, including country-specific and region-specific variations, is crucial to inform public health policy and resource allocation.
-
An understanding of DPN risk factors can guide treatment and prevention strategies, pivoting from purely glycaemic control to more multi-targeted metabolic control through diet and exercise lifestyle changes.
-
DPN education and awareness campaigns are needed to disseminate knowledge and encourage best clinical practice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Feldman, E. L. et al. Diabetic neuropathy. Nat. Rev. Dis. Prim. 5, 41 (2019).
Stino, A. M. & Smith, A. G. Peripheral neuropathy in prediabetes and the metabolic syndrome. J. Diabetes Investig. 8, 646–655 (2017).
Elafros, M. A. et al. Towards prevention of diabetic peripheral neuropathy: clinical presentation, pathogenesis, and new treatments. Lancet Neurol. 21, 922–936 (2022).
Hicks, C. W. et al. The association of peripheral neuropathy detected by monofilament testing with risk of falls and fractures in older adults. J. Am. Geriatr. Soc. 71, 1902–1909 (2023).
Gore, M. et al. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J. Pain. Symptom Manag. 30, 374–385 (2005).
Wei, K. S., Gu, M. Z., Zhu, J. W., Hu, H. C. & Yin, L. P. Current views of diabetic peripheral neuropathic pain comorbid depression – a review. Eur. Rev. Med. Pharmacol. Sci. 24, 10663–10670 (2020).
Boulton, A. J. M. et al. Diagnosis and Management of Diabetic Foot Complications (American Diabetes Association, 2018).
McDermott, K., Fang, M., Boulton, A. J. M., Selvin, E. & Hicks, C. W. Etiology, epidemiology, and disparities in the burden of diabetic foot ulcers. Diabetes Care 46, 209–221 (2023).
Vági, O. E. et al. The association between distal symmetric polyneuropathy in diabetes with all-cause mortality – a meta-analysis. Front. Endocrinol. 14, 1079009 (2023).
Hicks, C. W., Wang, D., Matsushita, K., Windham, B. G. & Selvin, E. Peripheral neuropathy and all-cause and cardiovascular mortality in U.S. adults: a prospective cohort study. Ann. Intern. Med. 174, 167–174 (2021).
Zhang, Y. et al. Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016. Diabetes Care 43, 964–974 (2020).
Ahmad, E., Lim, S., Lamptey, R., Webb, D. R. & Davies, M. J. Type 2 diabetes. Lancet 400, 1803–1820 (2022).
NCD Countdown 2030 Collaborators. NCD countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet 392, 1072–1088 (2018).
Sun, H. et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119 (2022).
GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402, 203–234 (2023).
Hansen, C. S., Määttä, L. L., Andersen, S. T. & Charles, M. H. in Diabetic Neuropathy: Advances in Pathophysiology and Clinical Management 3rd edn (eds Tesfaye, S. et al.) 5–36 (Springer, 2023).
Gylfadottir, S. S. et al. Diabetic polyneuropathy and pain, prevalence, and patient characteristics: a cross-sectional questionnaire study of 5,514 patients with recently diagnosed type 2 diabetes. Pain 161, 574–583 (2020).
Brask-Thomsen, P. K. et al. Development and progression of polyneuropathy over 5 years in patients with type 2 diabetes. Neurology 103, e209652 (2024).
Lu, Y. et al. Prevalence and risk factors for diabetic peripheral neuropathy in type 2 diabetic patients from 14 countries: estimates of the INTERPRET-DD study. Front. Public. Health 8, 534372 (2020).
Kosiborod, M. et al. Vascular complications in patients with type 2 diabetes: prevalence and associated factors in 38 countries (the DISCOVER study program). Cardiovasc. Diabetol. 17, 150 (2018).
Sun, J., Wang, Y., Zhang, X., Zhu, S. & He, H. Prevalence of peripheral neuropathy in patients with diabetes: a systematic review and meta-analysis. Prim. Care Diabetes 14, 435–444 (2020).
Shiferaw, W. S., Akalu, T. Y., Work, Y. & Aynalem, Y. A. Prevalence of diabetic peripheral neuropathy in Africa: a systematic review and meta-analysis. BMC Endocr. Disord. 20, 49 (2020).
Kibirige, D. et al. Indicators of optimal diabetes care and burden of diabetes complications in Africa: a systematic review and meta-analysis. BMJ Open. 12, e060786 (2022).
Aikaeli, F. et al. Prevalence of microvascular and macrovascular complications of diabetes in newly diagnosed type 2 diabetes in low-and-middle-income countries: a systematic review and meta-analysis. PLoS Glob. Public. Health 2, e0000599 (2022).
Yovera-Aldana, M. et al. Prevalence and incidence of diabetic peripheral neuropathy in Latin America and the Caribbean: a systematic review and meta-analysis. PLoS ONE 16, e0251642 (2021).
Hicks, C. W., Wang, D., Windham, B. G., Matsushita, K. & Selvin, E. Prevalence of peripheral neuropathy defined by monofilament insensitivity in middle-aged and older adults in two US cohorts. Sci. Rep. 11, 19159 (2021).
Jaiswal, M. et al. Prevalence of and risk factors for diabetic peripheral neuropathy in youth with type 1 and type 2 diabetes: SEARCH for Diabetes in Youth study. Diabetes Care 40, 1226–1232 (2017).
International Diabetes Federation. Russian Federation: Diabetes Report 2000–2045. IDF Diabetes Atlas diabetesatlas.org/data/en/country/166/ru.html (2021).
International Diabetes Federation. Egypt: Diabetes Report 2000–2045. IDF Diabetes Atlas diabetesatlas.org/data/en/country/61/eg.html (2021).
International Diabetes Federation. Australia: Diabetes Report 2000–2045. IDF Diabetes Atlas diabetesatlas.org/data/en/country/11/au.html (2021).
Zhang, P. et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann. Med. 49, 106–116 (2017).
Nascimento de Aquino, M. J. et al. Prevalence, incidence and factors associated with diabetic foot in people with type 2 diabetes: systematic review with meta-analysis. Curr. Diabetes Rev. 20, e070423215527 (2024).
Maldonado-Valer, T. et al. Prevalence of diabetic foot at risk of ulcer development and its components stratification according to the International Working Group on the Diabetic Foot (IWGDF): a systematic review with metanalysis. PLoS ONE 18, e0284054 (2023).
Ezzatvar, Y. & García-Hermoso, A. Global estimates of diabetes-related amputations incidence in 2010-2020: a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 195, 110194 (2023).
Hughes, W. et al. Editor’s choice – trends in lower extremity amputation incidence in European Union 15+ countries 1990–2017. Eur. J. Vasc. Endovasc. Surg. 60, 602–612 (2020).
Fu, X. L. et al. Global recurrence rates in diabetic foot ulcers: a systematic review and meta-analysis. Diabetes Metab. Res. Rev. 35, e3160 (2019).
Chen, L., Sun, S., Gao, Y. & Ran, X. Global mortality of diabetic foot ulcer: a systematic review and meta-analysis of observational studies. Diabetes Obes. Metab. 25, 36–45 (2023).
Rigato, M. et al. Characteristics, prevalence, and outcomes of diabetic foot ulcers in Africa. A systemic review and meta-analysis. Diabetes Res. Clin. Pract. 142, 63–73 (2018).
Abbas, Z. G. & Boulton, A. J. M. Diabetic foot ulcer disease in African continent: ‘From clinical care to implementation’ – review of diabetic foot in last 60 years – 1960 to 2020. Diabetes Res. Clin. Pract. 183, 109155 (2022).
Mairghani, M. et al. The prevalence and incidence of diabetic foot ulcers among five countries in the Arab world: a systematic review. J. Wound Care 26, S27–S34 (2017).
Zhang, Y. et al. Diabetes-related foot disease in Australia: a systematic review of the prevalence and incidence of risk factors, disease and amputation in Australian populations. J. Foot Ankle Res. 14, 8 (2021).
Finnerup, N. B. et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 14, 162–173 (2015).
Tesfaye, S. et al. Diagnosis, management and impact of painful diabetic peripheral neuropathy: a patient survey in four European countries. J. Diabetes Complications 37, 108417 (2023).
Naranjo, C. et al. Anxiety, depression and sleep disorders in patients with diabetic neuropathic pain: a systematic review. Expert. Rev. Neurother. 19, 1201–1209 (2019).
Pouwer, F. et al. Psychosocial care for people with diabetic neuropathy: time for action. Diabetes Care 47, 17–25 (2024).
Van Acker, K. et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 35, 206–213 (2009).
Tölle, T., Xu, X. & Sadosky, A. B. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J. Diabetes Complications 20, 26–33 (2006).
Bouhassira, D. et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 114, 29–36 (2005).
Sachau, J. et al. Patient reported outcome measures in chronic neuropathic pain clinical trials – a systematic literature review. J. Pain. 24, 38–54 (2023).
Jensen, T. S. et al. Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain 144, 1632–1645 (2021).
Hébert, H. L., Veluchamy, A., Torrance, N. & Smith, B. H. Risk factors for neuropathic pain in diabetes mellitus. Pain 158, 560–568 (2017).
Abbott, C. A., Malik, R. A., van Ross, E. R., Kulkarni, J. & Boulton, A. J. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 34, 2220–2224 (2011).
Gylfadottir, S. S. et al. The characteristics of pain and dysesthesia in patients with diabetic polyneuropathy. PLoS ONE 17, e0263831 (2022).
Themistocleous, A. C. et al. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain 157, 1132–1145 (2016).
Raputova, J. et al. Continuum of sensory profiles in diabetes mellitus patients with and without neuropathy and pain. Eur. J. Pain. 26, 2198–2212 (2022).
Baskozos, G. et al. Classification of painful or painless diabetic peripheral neuropathy and identification of the most powerful predictors using machine learning models in large cross-sectional cohorts. BMC Med. Inf. Decis. Mak. 22, 144 (2022).
Ziegler, D. et al. Painful and painless neuropathies are distinct and largely undiagnosed entities in subjects participating in an educational initiative (PROTECT study). Diabetes Res. Clin. Pract. 139, 147–154 (2018).
Allwright, M., Karrasch, J. F., O’Brien, J. A., Guennewig, B. & Austin, P. J. Machine learning analysis of the UK Biobank reveals prognostic and diagnostic immune biomarkers for polyneuropathy and neuropathic pain in diabetes. Diabetes Res. Clin. Pract. 201, 110725 (2023).
Åkerlund, M. et al. Genetic associations of neuropathic pain and sensory profile in a deeply phenotyped neuropathy cohort. Pain https://doi.org/10.1097/j.pain.0000000000003463 (2024).
Daousi, C., Benbow, S. J., Woodward, A. & MacFarlane, I. A. The natural history of chronic painful peripheral neuropathy in a community diabetes population. Diabet. Med. 23, 1021–1024 (2006).
Bommer, C. et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care 41, 963–970 (2018).
Pan, Q. et al. How does diabetic peripheral neuropathy impact patients’ burden of illness and the economy? A retrospective study in Beijing, China. Front. Public. Health 11, 1164536 (2023).
Zhao, Y. et al. Prevalence of other diabetes-associated complications and comorbidities and its impact on health care charges among patients with diabetic neuropathy. J. Diabetes Complications 24, 9–19 (2010).
Mehra, M., Merchant, S., Gupta, S. & Potluri, R. C. Diabetic peripheral neuropathy: resource utilization and burden of illness. J. Med. Econ. 17, 637–645 (2014).
Stewart, W. F., Ricci, J. A., Chee, E., Hirsch, A. G. & Brandenburg, N. A. Lost productive time and costs due to diabetes and diabetic neuropathic pain in the US workforce. J. Occup. Environ. Med. 49, 672–679 (2007).
Rice, J. B. et al. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care 37, 651–658 (2014).
Hicks, C. W. et al. The Society for Vascular Surgery Wound, Ischemia, and Foot Infection (WIfI) classification system correlates with cost of care for diabetic foot ulcers treated in a multidisciplinary setting. J. Vasc. Surg. 67, 1455–1462 (2018).
Kerr, M. et al. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet. Med. 36, 995–1002 (2019).
Callaghan, B. C., Little, A. A., Feldman, E. L. & Hughes, R. A. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst. Rev. 6, CD007543 (2012).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112, 2735–2752 (2005).
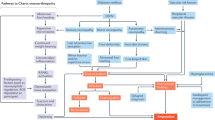
Grisold, A., Callaghan, B. C. & Feldman, E. L. Mediators of diabetic neuropathy: is hyperglycemia the only culprit? Curr. Opin. Endocrinol. Diabetes Obes. 24, 103–111 (2017).
Hanewinckel, R. et al. Metabolic syndrome is related to polyneuropathy and impaired peripheral nerve function: a prospective population-based cohort study. J. Neurol. Neurosurg. Psychiatry 87, 1336–1342 (2016).
Costa, L. A., Canani, L. H., Lisbôa, H. R., Tres, G. S. & Gross, J. L. Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in type 2 diabetes. Diabet. Med. 21, 252–255 (2004).
Han, L. et al. Peripheral neuropathy is associated with insulin resistance independent of metabolic syndrome. Diabetol. Metab. Syndr. 7, 14 (2015).
Callaghan, B. C. et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann. Clin. Transl. Neurol. 5, 397–405 (2018).
Callaghan, B. C. et al. Central obesity is associated with neuropathy in the severely obese. Mayo Clin. Proc. 95, 1342–1353 (2020).
Andersen, S. T. et al. Risk-factor trajectories preceding diabetic polyneuropathy: ADDITION-Denmark. Diabetes Care 41, 1955–1962 (2018).
Christensen, D. H. et al. Metabolic factors, lifestyle habits, and possible polyneuropathy in early type 2 diabetes: a nationwide study of 5,249 patients in the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort. Diabetes Care 43, 1266–1275 (2020).
Kapoor, N. et al. Consensus on medical nutrition therapy for diabesity (CoMeND) in adults: a South Asian perspective. Diabetes Metab. Syndr. Obes. 14, 1703–1728 (2021).
WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363, 157–163 (2004).
Misra, A. et al. Body fat, metabolic syndrome and hyperglycemia in South Asians. J. Diabetes Complications 32, 1068–1075 (2018).
Reynolds, E. L., Callaghan, B. C., Banerjee, M., Feldman, E. L. & Viswanathan, V. The metabolic drivers of neuropathy in India. J. Diabetes Complications 34, 107653 (2020).
Govindarajan Venguidesvarane, A. et al. Prevalence of vascular complications among type 2 diabetic patients in a rural health center in South India. J. Prim. Care Community Health 11, 2150132720959962 (2020).
Mathiyalagen, P., Kanagasabapathy, S., Kadar, Z., Rajagopal, A. & Vasudevan, K. Prevalence and determinants of peripheral neuropathy among adult type II diabetes mellitus patients attending a non-communicable disease clinic in rural South India. Cureus 13, e15493 (2021).
Srinivasan, S. et al. Four-year incident neuropathy and its risk factors in subjects with type 2 diabetes. J. Assoc. Physicians India 67, 34–37 (2019).
Wang, W. et al. Prevalence and risk factors of diabetic peripheral neuropathy: a population-based cross-sectional study in China. Diabetes Metab. Res. Rev. 39, e3702 (2023).
Alberti, K. G. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 (2009).
Eid, S. A. et al. New perspectives in diabetic neuropathy. Neuron 111, 2623–2641 (2023).
Rumora, A. E. et al. A high-fat diet disrupts nerve lipids and mitochondrial function in murine models of neuropathy. Front. Physiol. 13, 921942 (2022).
Grote, C. W. & Wright, D. E. A role for insulin in diabetic neuropathy. Front. Neurosci. 10, 581 (2016).
Cameron, N. E., Eaton, S. E., Cotter, M. A. & Tesfaye, S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 44, 1973–1988 (2001).
Eid, S. A., Savelieff, M. G., Eid, A. A. & Feldman, E. L. Nox, Nox, are you there? The role of NADPH oxidases in the peripheral nervous system. Antioxid. Redox Signal. 37, 613–630 (2022).
Saika, F., Kiguchi, N., Matsuzaki, S., Kobayashi, D. & Kishioka, S. Inflammatory macrophages in the sciatic nerves facilitate neuropathic pain associated with type 2 diabetes mellitus. J. Pharmacol. Exp. Ther. 368, 535–544 (2019).
Pop-Busui, R. et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 40, 136–154 (2017).
Martin, C. L., Albers, J. W. & Pop-Busui, R. Neuropathy and related findings in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Care 37, 31–38 (2014).
Corbin, K. D. et al. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr. Rev. 39, 629–663 (2018).
Lee, A. S., Twigg, S. M. & Flack, J. R. Metabolic syndrome in type 1 diabetes and its association with diabetes complications. Diabet. Med. 38, e14376 (2021).
Padda, I. S., Mahtani, A. U. & Parmar, M. Sodium–Glucose Transport Protein 2 (SGLT2) Inhibitors. (StatPearls, 2024).
Liao, J. et al. The impact of canagliflozin on the risk of neuropathy events: a post-hoc exploratory analysis of the CREDENCE trial. Diabetes Metab. 48, 101331 (2022).
Eid, S. A. et al. Differential effects of empagliflozin on microvascular complications in murine models of type 1 and type 2 diabetes. Biology 9, 347 (2020).
Collins, L. & Costello, R. A. Glucagon-Like Peptide-1 Receptor Agonists (StatPearls, 2024).
García-Casares, N. et al. Effects of GLP-1 receptor agonists on neurological complications of diabetes. Rev. Endocr. Metab. Disord. 24, 655–672 (2023).
Vandevijvere, S. et al. Global trends in ultraprocessed food and drink product sales and their association with adult body mass index trajectories. Obes. Rev. 20, 10–19 (2019).
Harb, A. A., Shechter, A., Koch, P. A. & St-Onge, M. P. Ultra-processed foods and the development of obesity in adults. Eur. J. Clin. Nutr. 77, 619–627 (2023).
Neri, D. et al. Ultraprocessed food consumption and dietary nutrient profiles associated with obesity: a multicountry study of children and adolescents. Obes. Rev. 23, e13387 (2022).
Miller, V. et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet 390, 2037–2049 (2017).
Schwingshackl, L. et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 32, 363–375 (2017).
Cooper, A. J. et al. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur. J. Clin. Nutr. 66, 1082–1092 (2012).
Jing, T. et al. Effect of dietary approaches on glycemic control in patients with type 2 diabetes: a systematic review with network meta-analysis of randomized trials. Nutrients 15, 3156 (2023).
Borgundvaag, E., Mak, J. & Kramer, C. K. Metabolic impact of intermittent fasting in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of interventional studies. J. Clin. Endocrinol. Metab. 106, 902–911 (2021).
Reynolds, E. L. et al. The effect of surgical weight loss on diabetes complications in individuals with class II/III obesity. Diabetologia 66, 1192–1207 (2023).
Müller-Stich, B. P. et al. Gastric bypass leads to improvement of diabetic neuropathy independent of glucose normalization – results of a prospective cohort study (DiaSurg 1 study). Ann. Surg. 258, 760–765 (2013).
Adam, S. et al. Improvements in diabetic neuropathy and nephropathy after bariatric surgery: a prospective cohort study. Obes. Surg. 31, 554–563 (2021).
Callaghan, B. C. et al. Dietary weight loss in people with severe obesity stabilizes neuropathy and improves symptomatology. Obesity 29, 2108–2118 (2021).
Romagnolo, D. F. & Selmin, O. I. Mediterranean diet and prevention of chronic diseases. Nutr. Today 52, 208–222 (2017).
Rumora, A. E. et al. The divergent roles of dietary saturated and monounsaturated fatty acids on nerve function in murine models of obesity. J. Neurosci. 39, 3770–3781 (2019).
Shevalye, H. et al. Effect of enriching the diet with menhaden oil or daily treatment with resolvin D1 on neuropathy in a mouse model of type 2 diabetes. J. Neurophysiol. 114, 199–208 (2015).
Zúnica-García, S., Blanquer-Gregori, J. J., Sánchez-Ortiga, R., Jiménez-Trujillo, M. I. & Chicharro-Luna, E. Relationship between diabetic peripheral neuropathy and adherence to the Mediterranean diet in patients with type 2 diabetes mellitus: an observational study. J. Endocrinol. Invest. 47, 2603–2613 (2024).
Dannawi, M. et al. Influence of intermittent fasting on prediabetes-induced neuropathy: insights on a novel mechanistic pathway. Metab. Open. 14, 100175 (2022).
Eid, S. A. et al. Dietary interventions improve diabetic kidney disease, but not peripheral neuropathy, in a db/db mouse model of type 2 diabetes. Faseb J. 37, e23115 (2023).
Kender, Z. et al. Six-month periodic fasting does not affect somatosensory nerve function in type 2 diabetes patients. Front. Endocrinol. 14, 1143799 (2023).
Li, R. J., Liu, Y., Liu, H. Q. & Li, J. Ketogenic diets and protective mechanisms in epilepsy, metabolic disorders, cancer, neuronal loss, and muscle and nerve degeneration. J. Food Biochem. 44, e13140 (2020).
Enders, J., Elliott, D. & Wright, D. E. Emerging nonpharmacologic interventions to treat diabetic peripheral neuropathy. Antioxid. Redox Signal. 38, 989–1000 (2023).
Enders, J., Swanson, M. T., Ryals, J. & Wright, D. E. A ketogenic diet reduces mechanical allodynia and improves epidermal innervation in diabetic mice. Pain 163, 682–689 (2022).
Cooper, M. A. et al. A ketogenic diet reduces metabolic syndrome-induced allodynia and promotes peripheral nerve growth in mice. Exp. Neurol. 306, 149–157 (2018).
Dyall, S. C. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 7, 52 (2015).
Tao, M., McDowell, M. A., Saydah, S. H. & Eberhardt, M. S. Relationship of polyunsaturated fatty acid intake to peripheral neuropathy among adults with diabetes in the National Health and Nutrition Examination Survey (NHANES) 1999 2004. Diabetes Care 31, 93–95 (2008).
Lauretani, F. et al. Omega-6 and omega-3 fatty acids predict accelerated decline of peripheral nerve function in older persons. Eur. J. Neurol. 14, 801–808 (2007).
Lewis, E. J. H. et al. Effect of omega-3 supplementation on neuropathy in type 1 diabetes: a 12-month pilot trial. Neurology 88, 2294–2301 (2017).
Durán, A. M., Beeson, W. L., Firek, A., Cordero-MacIntyre, Z. & De León, M. Dietary omega-3 polyunsaturated fatty-acid supplementation upregulates protective cellular pathways in patients with type 2 diabetes exhibiting improvement in painful diabetic neuropathy. Nutrients 14, 761 (2022).
Bull, F. C. et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 54, 1451–1462 (2020).
Guthold, R., Stevens, G. A., Riley, L. M. & Bull, F. C. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob. Health 6, e1077–e1086 (2018).
Zhao, X., He, Q., Zeng, Y. & Cheng, L. Effectiveness of combined exercise in people with type 2 diabetes and concurrent overweight/obesity: a systematic review and meta-analysis. BMJ Open. 11, e046252 (2021).
Ponirakis, G. et al. Progressive loss of corneal nerve fibers is associated with physical inactivity and glucose lowering medication associated with weight gain in type 2 diabetes. J. Diabetes Investig. 13, 1703–1710 (2022).
Lewis, E. J. H. et al. The association between physical activity time and neuropathy in longstanding type 1 diabetes: a cross-sectional analysis of the Canadian study of longevity in type 1 diabetes. J. Diabetes Complications 36, 108134 (2022).
Orlando, G. et al. Sedentary behaviour is an independent predictor of diabetic foot ulcer development: an 8-year prospective study. Diabetes Res. Clin. Pract. 177, 108877 (2021).
Vibha, S. P., Kulkarni, M. M., Kirthinath Ballala, A. B., Kamath, A. & Maiya, G. A. Community based study to assess the prevalence of diabetic foot syndrome and associated risk factors among people with diabetes mellitus. BMC Endocr. Disord. 18, 43 (2018).
Cooper, M. A. et al. Modulation of diet-induced mechanical allodynia by metabolic parameters and inflammation. J. Peripher. Nerv. Syst. 22, 39–46 (2017).
Tesfaye, S., Harris, N. D., Wilson, R. M. & Ward, J. D. Exercise-induced conduction velocity increment: a marker of impaired peripheral nerve blood flow in diabetic neuropathy. Diabetologia 35, 155–159 (1992).
Balducci, S. et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J. Diabetes Complications 20, 216–223 (2006).
Singleton, J. R. et al. Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Ann. Clin. Transl. Neurol. 1, 844–849 (2014).
Singleton, J. R., Marcus, R. L., Lessard, M. K., Jackson, J. E. & Smith, A. G. Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann. Neurol. 77, 146–153 (2015).
Smith, A. G. et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care 29, 1294–1299 (2006).
Dixit, S., Maiya, A. G. & Shastry, B. A. Effect of aerobic exercise on peripheral nerve functions of population with diabetic peripheral neuropathy in type 2 diabetes: a single blind, parallel group randomized controlled trial. J. Diabetes Complications 28, 332–339 (2014).
Khan, K. S. et al. Effects of progressive resistance training in individuals with type 2 diabetic polyneuropathy: a randomised assessor-blinded controlled trial. Diabetologia 65, 620–631 (2022).
Bönhof, G. J. et al. High-intensity interval training for 12 weeks improves cardiovascular autonomic function but not somatosensory nerve function and structure in overweight men with type 2 diabetes. Diabetologia 65, 1048–1057 (2022).
Stubbs, E. B. Jr et al. Randomized controlled trial of physical exercise in diabetic veterans with length-dependent distal symmetric polyneuropathy. Front. Neurosci. 13, 51 (2019).
Streckmann, F. et al. Exercise and neuropathy: systematic review with meta-analysis. Sports Med. 52, 1043–1065 (2022).
Tatikola, S. P., Natarajan, V., Desai, V. K., Asirvatham, A. R. & Rajsekhar, H. Effect of various exercise protocols on neuropathic pain in individuals with type 2 diabetes with peripheral neuropathy: a systematic review and meta-analysis. Diabetes Metab. Syndr. 16, 102603 (2022).
Braveman, P. & Gottlieb, L. The social determinants of health: it’s time to consider the causes of the causes. Public. Health Rep. 129, 19–31 (2014).
Kind, A. J. H. & Buckingham, W. R. Making neighborhood-disadvantage metrics accessible – the Neighborhood Atlas. N. Engl. J. Med. 378, 2456–2458 (2018).
Höhn, A. et al. Large socioeconomic gap in period life expectancy and life years spent with complications of diabetes in the Scottish population with type 1 diabetes, 2013–2018. PLoS ONE 17, e0271110 (2022).
Kurani, S. S. et al. Association of area-level socioeconomic deprivation with hypoglycemic and hyperglycemic crises in US adults with diabetes. JAMA Netw. Open. 5, e2143597 (2022).
Kurani, S. S. et al. Association between area-level socioeconomic deprivation and diabetes care quality in US primary care practices. JAMA Netw. Open. 4, e2138438 (2021).
Cuddapah, G. V. et al. Complications in diabetes mellitus: social determinants and trends. Cureus 14, e24415 (2022).
Jeyam, A. et al. Diabetic neuropathy is a substantial burden in people with type 1 diabetes and is strongly associated with socioeconomic disadvantage: a population-representative study from Scotland. Diabetes Care 43, 734–742 (2020).
Collier, A., Ghosh, S., Hair, M. & Waugh, N. Impact of socioeconomic status and gender on glycaemic control, cardiovascular risk factors and diabetes complications in type 1 and 2 diabetes: a population based analysis from a Scottish region. Diabetes Metab. 41, 145–151 (2015).
Ha, J. H., Jin, H. & Park, J. U. Association between socioeconomic position and diabetic foot ulcer outcomes: a population-based cohort study in South Korea. BMC Public. Health 21, 1395 (2021).
Herder, C. et al. Environmental risk factors of incident distal sensorimotor polyneuropathy: results from the prospective population-based KORA F4/FF4 study. Sci. Total. Environ. 858, 159878 (2023).
Elafros, M. A. et al. Patient and health care provider knowledge of diabetes and diabetic microvascular complications: a comprehensive literature review. Rev. Endocr. Metab. Disord. 24, 221–239 (2023).
Li, R. et al. The current status of foot self-care knowledge,behaviours, and analysis of influencing factors in patients with type 2 diabetes mellitus in China. Int. J. Nurs. Sci. 1, 226–271 (2014).
Zhu, W. et al. Relationship between diabetic knowledge, attitudes and practices among patients with diabetes in China: a structural equation model. BMJ Open. 13, e076464 (2023).
Frykberg, R. G., Vileikyte, L., Boulton, A. J. M. & Armstrong, D. G. The at-risk diabetic foot: time to focus on prevention. Diabetes Care 45, e144–e145 (2022).
Goodall, R. J. et al. A systematic review of the impact of foot care education on self efficacy and self care in patients with diabetes. Eur. J. Vasc. Endovasc. Surg. 60, 282–292 (2020).
Boulton, A. J. M. et al. New Evidence-Based Therapies for Complex Diabetic Foot Wounds (American Diabetes Association, 2022).
Suglo, J. N., Winkley, K. & Sturt, J. Prevention and management of diabetes-related foot ulcers through informal caregiver involvement: a systematic review. J. Diabetes Res. 2022, 9007813 (2022).
Daly, B., Arroll, B., Sheridan, N., Kenealy, T. & Scragg, R. Diabetes knowledge of nurses providing community care for diabetes patients in Auckland, New Zealand. Prim. Care Diabetes 8, 215–223 (2014).
Faselis, C. et al. Microvascular complications of type 2 diabetes mellitus. Curr. Vasc. Pharmacol. 18, 117–124 (2020).
Sadosky, A., Hopper, J. & Parsons, B. Painful diabetic peripheral neuropathy: results of a survey characterizing the perspectives and misperceptions of patients and healthcare practitioners. Patient 7, 107–114 (2014).
O’Brien, S. V., Michaels, S. E. & Hardy, K. J. A comparison of general nurses’ and junior doctors’ diabetes knowledge. Professional Nurse 18, 257–260 (2003).
Wang, H. et al. Knowledge, attitudes, and practice of endocrinology healthcare workers regarding screening for pre-ulcerative diabetic foot lesions. J. Tissue Viability 32, 472–479 (2023).
Alsheikh, S. et al. Diabetic foot care: a screening on primary care providers’ attitude and practice in Riyadh, Saudi Arabia. Medicina 59, 64 (2022).
Liu, G. et al. Adherence to a healthy lifestyle in association with microvascular complications among adults with type 2 diabetes. JAMA Netw. Open. 6, e2252239 (2023).
Andersen, S. T. et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care 41, 1068–1075 (2018).
Kirthi, V. et al. Prevalence of peripheral neuropathy in pre-diabetes: a systematic review. BMJ Open. Diabetes Res. Care 9, e002040 (2021).
Boulton, A. Protect tomorrow by investing in diabetes education today. Diabetes Res Clin Pract. 194, 110180 (2022).
International Diabetes Federation. Nigeria: Diabetes Report 2000–2045. IDF Diabetes Atlas diabetesatlas.org/data/en/country/145/ng.html (2021).
Bello, A., Biliaminu, S., Wahab, K. & Sanya, E. Distal symmetrical polyneuropathy and cardiovascular autonomic neuropathy among diabetic patients in Ilorin: prevalence and predictors. Niger. Postgrad. Med. J. 26, 123–128 (2019).
Ikem, R. T., Enikuomehin, A. C., Soyoye, D. O. & Kolawole, B. A. The burden of diabetic complications in subjects with type 2 diabetes attending the diabetes clinic of the Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria – a cross-sectional study. Pan Afr. Med. J. 43, 148 (2022).
Anumah, F. E. et al. Common and contrast determinants of peripheral artery disease and diabetic peripheral neuropathy in North Central Nigeria. Foot 55, 101987 (2023).
Aliyu, R., Gezawa, I. D., Uloko, A. E. & Ramalan, M. A. Prevalence and risk factors of diabetes foot ulcers in Kano, northwestern Nigeria. Clin. Diabetes Endocrinol. 9, 6 (2023).
Salawu, A. I. et al. Diabetes mellitus foot ulcer and associated factors among type 2 diabetes patients in a tertiary institution in Southwest Nigeria. Ann. Afr. Med. 21, 339–347 (2022).
International Diabetes Federation. India: Diabetes Report 2000–2045. IDF Diabetes Atlas diabetesatlas.org/data/en/country/93/in.html (2021).
Pradeepa, R. et al. Sex-based differences in clinical profile and complications among individuals with type 2 diabetes seen at a private tertiary diabetes care centre in India. Healthcare 11, 1634 (2023).
Pradeepa, R. & Mohan, V. Prevalence of type 2 diabetes and its complications in India and economic costs to the nation. Eur. J. Clin. Nutr. 71, 816–824 (2017).
Patil, S. S. et al. A prospective study on type-2 diabetic complications and efficacy of integrated yoga: a pan India 2017. Ann. Neurosci. 28, 21–28 (2021).
Jadhao, P., Swain, J., Das, S., Mangaraj, S. & Sravya, S. L. Prevalence and predictors of diabetic peripheral neuropathy in newly diagnosed type 2 diabetes mellitus patients. Curr. Diabetes Rev. https://doi.org/10.2174/0115733998282818240125110248 (2024).
Rastogi, A. et al. Long term outcomes after incident diabetic foot ulcer: multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study: epidemiology of diabetic foot complications study. Diabetes Res. Clin. Pract. 162, 108113 (2020).
Kumpatla, S., Kothandan, H., Tharkar, S. & Viswanathan, V. The costs of treating long-term diabetic complications in a developing country: a study from India. J. Assoc. Physicians India 61, 102–109 (2013).
Viswanathan, V. et al. Profile of diabetic foot complications and its associated complications – a multicentric study from India. J. Assoc. Physicians India 53, 933–936 (2005).
Viswanathan, V. & Kumpatla, S. Pattern and causes of amputation in diabetic patients – a multicentric study from India. J. Assoc. Physicians India 59, 148–151 (2011).
Viswanathan, V. & Rao, V. N. Managing diabetic foot infection in India. Int. J. Low. Extrem. Wounds 12, 158–166 (2013).
International Diabetes Federation. China: Diabetes Report 2000–2045. IDF Diabetes Atlas diabetesatlas.org/data/en/country/42/cn.html (2021).
Liu, J. et al. Risk factors for diabetic peripheral neuropathy, peripheral artery disease, and foot deformity among the population with diabetes in Beijing, China: a multicenter, cross-sectional study. Front. Endocrinol. 13, 824215 (2022).
Acknowledgements
The authors thank the study participants in the clinical studies that they led. They also thank E. J. Koubek for general assistance. M.G.S. acknowledges funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; grant R01DK130913). M.A.E acknowledges funding from the National Institute of Neurological Disorders and Stroke (grants K23NS131444 and R25NS089450) and the Andrea and Lawrence A. Wolfe Research Professorship. D.L.B. acknowledges funding from the Wellcome Trust (grant 223149/Z/21/Z), the UK Medical Research Council (grant MR/T020113/1), the UK Medical Research Council and Versus Arthritis to the PAINSTORM consortium as part of the Advanced Pain Discovery Platform (grant MR/W002388/1), the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 633491 (DOLORisk), and the Oxford Health Biomedical Research Centre. T.S.J., D.L.B., and E.L.F. were funded by the Novo Nordisk foundation as part of the International Diabetic Neuropathy Consortium (grant NNF14OC0011633). E.L.F acknowledges funding from the NIDDK (grants R01DK130913, R01DK129320 and R01DK107956), JDRF 5COE-2019-861-S-B, the Dr. John H. Doran Neuropathy Research Initiative, the Richard and Jane Manoogian Foundation, the Nathan and Rose Milstein Research Fund, the Sinai Medical Staff Foundation, the A. Alfred Taubman Medical Research Institute and the NeuroNetwork for Emerging Therapies.
Author information
Authors and Affiliations
Contributions
M.G.S. and D.L.B. researched data for the article. M.G.S. and E.L.F. contributed substantially to discussion of the article content. All authors wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T.S.J. has acted as consultant for Alnylam, Vectura, and the International Diabetic Federation. D.L.B. has acted as a consultant in the last 2 years for AditumBio, Amgen, Biogen, Biointervene, Combigene, LatigoBio, GSK, Ionis, Lexicon therapeutics, Lilly, Neuvati, Novo Ventures, Orion, Replay, SC Health Managers, Third Rock Ventures, and Vida Ventures. He has received research funding from Eli Lilly and Astra Zeneca. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks A. Eid, S. Yagihashi, C. Sommer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
For this scoping review, we searched PubMed for articles published in the English language from 1 January 2023 to 7 December 2023; however, older seminal papers were also considered, along with articles from the authors’ personal reference lists. In combination with “diabetic peripheral neuropathy” or “diabetic foot ulcer” we used one or more of the following terms: “Africa”, “America”, “Asia”, “Caribbean”, “China”, “clinical trial”, “cost”, “diet”, “dietary habit”, “dietary intervention”, “Europe”, “exercise”, “fruits”, “global”, “human”, “India”, “intermittent fasting”, “KAP”, “ketogenic diet”, “knowledge attitudes practices”, “lost productive time”, “Mediterranean diet”, “meta-analysis”, “metabolic syndrome”, “Middle-East”, “neuropathic pain”, “Nigeria”, “painful diabetic neuropathy”, “prevalence”, “sedentary”, “socioeconomic factors”, “trends”, “ultraprocessed foods”, “vegetables”, “Western Pacific” and “workforce”. Articles were selected on the basis of relevance to the Review topic, with an emphasis on meta-analyses to present the sum of the available data.
Supplementary information
Glossary
- Allostatic load
-
Damage caused to the body from the cumulative burden of chronic stress from life events.
- Chronic neuropathic pain
-
Chronic pain caused by a lesion or disease affecting the somatosensory system, now included in the International Classification of Disease 11th revision (code MG30.5).
- Intraepidermal nerve fibre density
-
(IENFD). A measure of small-fibre numbers. Small-fibre counts are made by immunohistochemically assessing epidermal tissue collected by skin punch biopsy. IENFD of the distal leg is reduced in patients with diabetic peripheral neuropathy (DPN).
- Large-fibre neuropathy
-
Injury to large myelinated fibres that induces generalized numbness with loss of vibratory and position sense.
- Monofilament testing
-
A technique for measuring large-fibre function in which light-touch pressure perception is assessed by pressing a nylon filament to the big toe.
- Nerve conduction velocity
-
(NCV). Electrophysiological measurements of conduction velocity of nerve impulses to assess large-fibre function. NCVs of peripheral nerves are reduced in patients with DPN.
- Prediabetes
-
A state preceding frank type 2 diabetes, characterized by insulin resistance, glucose intolerance and elevated fasting blood glucose that does not meet the formal criteria for diabetes.
- Quantitative sensory testing
-
A standardized battery of clinical tests that assess various sensory modalities, including pain, thermal or mechanical perception, hyperalgesia and allodynia. Quantitative sensory testing assesses both small-fibre and large-fibre neuropathy.
- Small-fibre neuropathy
-
Injury to small unmyelinated C and thinly myelinated Aδ fibres, which induces predominantly painful sensations, including prickling, burning and electric shock-like sensations.
- Type 1 diabetes
-
Diabetes resulting from autoimmune-mediated destruction of insulin-producing pancreatic β cells, leading to hyperglycaemia.
- Type 2 diabetes
-
Diabetes resulting from increased insulin resistance, leading to hyperglycaemia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Savelieff, M.G., Elafros, M.A., Viswanathan, V. et al. The global and regional burden of diabetic peripheral neuropathy. Nat Rev Neurol 21, 17–31 (2025). https://doi.org/10.1038/s41582-024-01041-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-024-01041-y
This article is cited by
-
Diabetic Peripheral Neuropathy: New Diagnostics and Treatment Perspectives
Drugs & Aging (2026)
-
Multimodal computational approaches coupled with experimental assays to identify flavonoids as potent inhibitors of diabetes and AGEs
Journal of Computer-Aided Molecular Design (2026)
-
Cactus Thorn-Inspired Janus Nanofiber Membranes as a Water Diode for Light-Enhanced Diabetic Wound Healing
Nano-Micro Letters (2026)
-
Advanced wound healing with Stimuli-Responsive nanozymes: mechanisms, design and applications
Journal of Nanobiotechnology (2025)
-
Machine learning in the prediction of diabetic peripheral neuropathy: a systematic review
BMC Medical Informatics and Decision Making (2025)