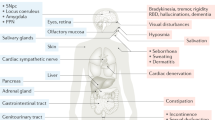
Abstract
The value of involving people living with diseases in the research process is increasingly recognized by professional associations and regulatory agencies alike. Patient contributions range from disease prevention and diagnosis to medication planning, and from advocacy to guideline production and clinical trial design. Thanks to the efforts of activists and advocates, new models of patient inclusion in medical research are being developed to replace outdated non-participative and tokenistic paradigms. New modalities of patient participation in research — for example, the introduction of patient experts, who work closely with researchers and clinicians — have progressively empowered individuals who are living with diseases. In this Perspective, we provide an overview of the current status of patient involvement in medical research, with a specific focus on neurology. We also discuss the existing and future roles of patient experts in neurological research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schroeder, K. et al. Building from patient experiences to deliver patient-focused healthcare systems in collaboration with patients: a call to action. Ther. Innov. Regul. Sci. 56, 848–858 (2022).
World Health Organization. Health 2020: A European Policy Framework and Strategy for the 21st Century (WHO, 2013).
Fox, G. et al. Patient engagement in preclinical laboratory research: a scoping review. eBioMedicine 70, 103484 (2021).
Geissler, J., Ryll, B., di Priolo, S. L. & Uhlenhopp, M. Improving patient involvement in medicines research and development: a practical roadmap. Ther. Innov. Regul. Sci. 51, 612–619 (2017).
National Institute for Health and Care Research. Briefing notes for researchers - public involvement in NHS, health and social care research. https://www.nihr.ac.uk/documents/briefing-notes-for-researchers-public-involvement-in-nhs-health-and-social-care-research/27371 (2024).
Marzban, S., Najafi, M., Agolli, A. & Ashrafi, E. Impact of patient engagement on healthcare quality: a scoping review. J. Patient Exp. https://doi.org/10.1177/23743735221125439 (2022).
Greenhalgh, T. et al. Frameworks for supporting patient and public involvement in research: systematic review and co‐design pilot. Health Expect. 22, 785–801 (2019).
Arnstein, S. R. A ladder of citizen participation. J. Am. Inst. Plann. 35, 216–224 (1969).
Timmermann, C. ‘Just give me the best quality of life questionnaire’: the Karnofsky scale and the history of quality of life measurements in cancer trials. Chronic Illn. 9, 179–190 (2013).
Wachter, R. M. AIDS, activism, and the politics of health. N. Engl. J. Med. 326, 128–133 (1992).
Colvin, C. J. Evidence and AIDS activism: HIV scale-up and the contemporary politics of knowledge in global public health. Glob. Public. Health 9, 57–72 (2014).
Osuch, J. R. et al. A historical perspective on breast cancer activism in the United States: from education and support to partnership in scientific research. J. Women’s Health 21, 355–362 (2012).
Landzelius, K. Introduction: patient organization movements and new metamorphoses in patienthood. Soc. Sci. Med. 62, 529–537 (2006).
Domecq, J. P. et al. Patient engagement in research: a systematic review. BMC Health Serv. Res. 14, 89 (2014).
Woudstra, K., Reuzel, R., Rovers, M. & Tummers, M. An overview of stakeholders, methods, topics, and challenges in participatory approaches used in the development of medical devices: a scoping review. Int. J. Health Policy Manag. 12, 6839 (2023).
Wellcome Trust. Community Engagement — Under the Microscope (Wellcome Trust, 2011).
World Health Organization. Intersectoral Global Action Plan on Epilepsy and Other Neurological Disorders (WHO, 2023).
Grisold, W., Struhal, W. & Grisold, T. in Advocacy in Neurology (Oxford University Press, 2019).
Winker, M. A. Tacrine for Alzheimer’s disease. Which patient, what dose? JAMA 271, 1023–1024 (1994).
Mattingly, T. J. & Simoni-Wastila, L. Patient-centered drug approval: the role of patient advocacy in the drug approval process. J. Manag. Care Spec. Pharm. 23, 1078–1082 (2017).
Mahant, V. “Right-to-Try” experimental drugs: an overview. J. Transl. Med. 18, 253 (2020).
Fournier, C. N. Considerations for amyotrophic lateral sclerosis (ALS) clinical trial design. Neurotherapeutics 19, 1180–1192 (2022).
Schnell, J. et al. ALS Association Ice Bucket Challenge Impact (RTI International, 2024).
European Federation of Neurological Associations. Optimising Patient Involvement in Neuroscience Research (EFNA, 2020).
Miah, J. et al. Patient and public involvement in dementia research in the European Union: a scoping review. BMC Geriatr. 19, 220 (2019).
Alzheimer’s Research UK. Other Ways to Get Involved. https://www.alzheimersresearchuk.org/research/getting-involved-in-research/other-ways-to-get-involved/ (2023).
Multiple Sclerosis Society. MS Society Research Strategy 2018–22 (MS Society, 2022).
Joyce, R., Dwyer, C. P. & Hynes, S. M. Twelve months into a feasibility trial: reflections on three experiences of public and patient involvement in research. HRB Open. Res. 4, 11 (2021).
Arumugam, A. et al. Patient and public involvement in research: a review of practical resources for young investigators. BMC Rheumatol. 7, 2 (2023).
Genuis, S. K. et al. Patient engagement in research: lessons learned from CAPTURE ALS, a longitudinal observational ALS study. Amyotroph. Lateral Scler. Front. Degener. 25, 634–643 (2024).
Berry, J. D., Bedlack, R., Mathews, D., Agnese, W. & Apple, S. Engaging ALS patients and caregivers (the ALS research ambassadors) to help design the REFINE-ALS biomarker study. Amyotroph. Lateral Scler. Front. Degener. 22, 147–150 (2021).
Levitan, B. et al. Assessing the financial value of patient engagement: a quantitative approach from CTTI’s Patient Groups and Clinical Trials project. Ther. Innov. Regul. Sci. 52, 220–229 (2018).
Reeves, M. et al. Patient-reported outcome measures (PROMs) for acute stroke: rationale, methods and future directions. Stroke 49, 1549–1556 (2018).
Alpuente, A., Gallardo, V. J., Caronna, E., Torres-Ferrus, M. & Pozo-Rosich, P. In search of a gold standard patient-reported outcome measure to use in the evaluation and treatment-decision making in migraine prevention. A real-world evidence study. J. Headache Pain. 22, 151 (2021).
Panthagani, J. et al. Evaluating patient-reported outcome measures (PROMs) for future clinical trials in adult patients with optic neuritis. Eye Lond. Engl. 37, 3097–3107 (2023).
Zaratin, P. et al. The agenda of the global patient reported outcomes for multiple sclerosis (PROMS) initiative: progresses and open questions. Mult. Scler. Relat. Disord. 61, 103757 (2022).
Schmahmann, J. D., Pierce, S., MacMore, J. & L’Italien, G. J. Development and validation of a patient‐reported outcome measure of ataxia. Mov. Disord. 36, 2367–2377 (2021).
Morel, T. et al. The value of co-creating a clinical outcome assessment strategy for clinical trial research: process and lessons learnt. Res. Involv. Engagem. 9, 98 (2023).
Morel, T. et al. Development and early qualitative evidence of two novel patient-reported outcome instruments to assess daily functioning in people with early-stage Parkinson’s. J. Patient Rep. Outcomes 7, 40 (2023).
Smith, M. Y. et al. Patients as research partners in preference studies: learnings from IMI-PREFER. Res. Involv. Engagem. 9, 21 (2023).
Eccles, M. P., Grimshaw, J. M., Shekelle, P., Schünemann, H. J. & Woolf, S. Developing clinical practice guidelines: target audiences, identifying topics for guidelines, guideline group composition and functioning and conflicts of interest. Implement. Sci. 7, 60 (2012).
NICE. Patient and Public Involvement Policy. https://www.nice.org.uk/about/nice-communities/nice-and-the-public/public-involvement/public-involvement-programme/patient-public-involvement-policy (2013).
EAN. About EAN Guidelines for Neurologists. https://www.ean.org/research/ean-guidelines/about-ean-guidelines-for-neurologists (2024).
Holloway, R. G., Benesch, C. & Rush, S. R. Stroke prevention: narrowing the evidence–practice gap. Neurology 54, 1899–1906 (2000).
World Stroke Organization. Stroke Advocacy Brochure (World Stroke Organization, 2022).
Wicks, P. Patient, study thyself. BMC Med. 16, 217 (2018).
Wicks, P., Vaughan, T. E., Massagli, M. P. & Heywood, J. Accelerated clinical discovery using self-reported patient data collected online and a patient-matching algorithm. Nat. Biotechnol. 29, 411–414 (2011).
Brownstein, C. A., Brownstein, J. S., Williams, D. S., Wicks, P. & Heywood, J. A. The power of social networking in medicine. Nat. Biotechnol. 27, 888–890 (2009).
Al-Chalabi, A. et al Lithium in patients with amyotrophic lateral sclerosis (LiCALS): a phase 3 multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 12, 339–345 (2013).
Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38, 101019 (2021).
Riggare, S. & Hägglund, M. Precision medicine in Parkinson’s disease — exploring patient-initiated self-tracking. J. Park. Dis. 8, 441–446 (2018).
Subramanian, I. et al. Unmet needs of women living with Parkinson’s disease: gaps and controversies. Mov. Disord. 37, 444–455 (2022).
Law, N., Davio, K., Blunck, M., Lobban, D. & Seddik, K. The lived experience of myasthenia gravis: a patient-led analysis. Neurol. Ther. 10, 1103–1125 (2021).
Woolley, K. L. et al. Patient authorship of medical research publications: an evolution, revolution, and solution? Learn. Publ. 37, e1607 (2024).
Chawla, D. S. Editors give green light to patients co-authoring manuscripts. Nature Index, https://www.nature.com/nature-index/news/editors-give-green-light-to-patients-co-authoring-manuscripts (2021).
Dahlberg, M. et al. Objectives and outcomes of patient-driven innovations published in peer-reviewed journals: a qualitative analysis of publications included in a scoping review. BMJ Open. 13, e071363 (2023).
Lewis, D. in Personal Health Informatics: Patient Participation in Precision Health (eds Hsueh, P. Y. S., Wetter, T., & Zhu, X.) (Springer, 2022).
Wannheden, C. et al. A rocky road but worth the drive: a longitudinal qualitative study of patient innovators and researchers cocreating research. Health Expect. 26, 1757–1767 (2023).
Reinius, M. et al. Patient-driven innovations reported in peer-reviewed journals: a scoping review. BMJ Open. 12, e053735 (2022).
Lewis, D., Leibrand, S. & OpenAPS Community. Real-world use of open source artificial pancreas systems. J. Diabetes Sci. Technol. 10, 1411 (2016).
FDA. CDER Patient-Focused Drug Development. https://www.fda.gov/drugs/development-approval-process-drugs/cder-patient-focused-drug-development (2024).
European Medicines Agency. Getting Involved in EMA Activities as a Patient, Consumer or Career. https://www.ema.europa.eu/en/partners-networks/patients-and-consumers/getting-involved (2023).
Regulation (EU) 2021/2282 of the European Parliament and of the Council of 15 December 2021 on health technology assessment and amending directive 2011/24/EU. European Union. https://eur-lex.europa.eu/eli/reg/2021/2282/oj (2021).
Meinders, M. J., Donnelly, A. C., Sheehan, M. & Bloem, B. R. Including people with Parkinson’s disease in clinical study design and execution: a call to action. J. Park. Dis. 12, 1359–1363 (2022).
Lithander, F. E. et al. Working with public contributors in Parkinson’s research: what were the changes, benefits and learnings? A critical reflection from the researcher and public contributor perspective. Health Expect. 27, e13914 (2024).
Moro, E. How can we stop digital technologies from worsening existing health inequalities? Nat. Rev. Neurol. 19, 449–450 (2023).
Riggare, S., Stecher, B. & Stamford, J. Patient advocates respond to ‘Utilizing Patient Advocates…’ by Feeney et al. Health Expect. 23, 972–973 (2020).
Aguzzoli Peres, F. et al. Walking the talk for dementia: a unique immersive, embodied, and multi‐experiential initiative. Alzheimers Dement. 20, 2309–2322 (2024).
World Health Organization. Declaration of Alma-Ata (WHO, 1978).
Fayers, P. MRC quality of life studies using a daily diary card—practical lessons learned from cancer trials. Qual. Life Res. 4, 343–352 (1995).
Merson, M. H., O’Malley, J., Serwadda, D. & Apisuk, C. The history and challenge of HIV prevention. Lancet 372, 475–488 (2008).
CDC. About the Prevention Research Centers. https://www.cdc.gov/prevention-research-centers/php/about/ (2024).
FDA. Evolution of Patient Engagement at the FDA. https://www.fda.gov/patients/evolution-patient-engagement-fda (2021).
INVOLVE Coordinating Centre. Starting out: essential information for members of the public getting started in involvement in research (NHS National Institute for Health Research, 2017).
Charlton, J. I. in Nothing About Us Without Us: Disability Oppression and Empowerment (University of California Press, 1998).
Donaldson, L. Expert patients usher in a new era of opportunity for the NHS. BMJ 326, 1279–1280 (2003).
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research., U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research & U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual. Life Outcomes 4, 79 (2006).
European Organisation for Rare Diseases. EURORDIS position paper on research priorities for rare diseases (EURORDIS — Rare Diseases Europe, 2009).
Canadian Institutes of Health Research. Strategy for Patient-Oriented Research. http://www.cihr-irsc.gc.ca/e/41204.html (2023).
NIHR. Improving Inclusion of Under-Served Groups in Clinical Research: Guidance from INCLUDE Project. https://www.nihr.ac.uk/improving-inclusion-under-served-groups-clinical-research-guidance-include-project (2024).
Post, A. E. M. et al. Research priorities for rare neurological diseases: a representative view of patient representatives and healthcare professionals from the European Reference Network for Rare Neurological Diseases. Orphanet J. Rare Dis. 16, 135 (2021).
European Medicines Agency. EMA Regulatory Science to 2025 (EMA, 2018).
Karagianni, E. Call for EoI for the IMI pool of patient experts. Innovative Medicines Initiative. https://www.ihi.europa.eu/sites/default/files/uploads/documents/get-involved/patients/PatientExpertWebinar.pdf (2019).
European Commission. Horizon 2020. https://research-and-innovation.ec.europa.eu/funding/funding-opportunities/funding-programmes-and-open-calls/horizon-2020_en (2024).
FDA. Plan for Issuance of Patient‐Focused Drug Development Guidance (FDA, 2017).
FDA. FDA patient-focused drug development guidance series for enhancing the incorporation of the patient’s voice in medical product development and regulatory decision making. https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical (2023).
European Medicines Agency & FDA. Terms of Reference for the EMA/FDA Cluster on Patient Engagement (EMA & FDA, 2016).
World Health Organization. Optimizing Brain Health Across the Life Course: WHO Position Paper (WHO, 2022).
Ruiz Gonzalez, F. et al. Parkinson disease in women: how does sex affect motor and non-motor symptoms? J. Park. Dis. 13, 66 (2023).
Mathur, S., Kristi Lamonica, K., Hill, K. & Matthews, H. Comprehensive survey data set of women with Parkisnon’s by women with Parkinson’s. J. Park. Dis. 13, 373 (2023).
Acknowledgements
The authors would like to thank Gloria Traina for her expert advice on regulatory and reimbursement processes, and Magdalena Eitenberger and Joann Leeding for useful discussions. The authors also thank the people with lived experience, family members and caregivers who have collaborated with us and inspired our work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.T.F. is the co-founder of the Women’s Brain Project. In the past 2 years she has received consulting and speaking fees from Roche, Angelini Pharma and Prodeco Pharma unrelated to this project. She is currently the Clinical Development Lead of Syntropic Medical. M.U. has received honoraria for consulting services from Alexion, Janssen, Merck, UCB Pharma and UCB S.A., and speaking fees from Alexion and UCB S.A.. She also provided consulting services to Argenx. Travel expenses for congresses were paid by Alexion, UCB Pharma, UCB S.A. and Argenx. R.F. is the co-founder and chief executive officer of My Moves Matter. E.M. has received restricted research grant support from Abbott and France Parkinson. She has also received honoraria from Medtronic for consultation services. I.L. declares advisory boards and honoraria for talks from Roche, NovoNordisk, Eli Lilly and Boehringer.
Peer review
Peer review information
Nature Reviews Neurology thanks C. McDermott, who co-reviewed with C. Massey; and M. Meinders for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Alzheimer’s Drug Discovery Foundation Diagnostic Accelerator: https://www.alzdiscovery.org/research-and-grants/diagnostics-accelerator
Alzheimer’s Research UK: https://www.alzheimersresearchuk.org/
Chan Zuckerberg Initiative Rare as One project: https://chanzuckerberg.com/science/programs-resources/rare-as-one/
Critical Path Institute: https://c-path.org/about/
European Organisation for Rare Diseases (EURORDIS) — Rare Diseases Europe: https://www.eurordis.org/
European Patients Academy on Therapeutic Innovation (EUPATI): https://eupati.eu/
My Moves Matter: https://www.mymovesmatter.com/
Patient-Centered Outcomes Research Institute: https://www.pcori.org/about/about-pcori
Patient Expert Training Programme: https://learning.eupati.eu/mod/page/view.php?id=1075&forceview=1
Patient-Led Research Collaborative: https://patientresearchcovid19.com/
PatientsLikeMe: https://www.patientslikeme.com/
The Michael J. Fox Foundation: https://www.michaeljfox.org/
Women’s Parkinson’s Project: https://www.womensparkinsonsproject.com/
Workgroup of European Cancer Patient Advocacy Networks: https://wecanadvocate.eu/
Glossary
- Citizen science
-
Any activity that involves the public in scientific research and generates genuine scientific outcomes, from the design of the research question, through data collection and volunteer mapping, data interpretation and analysis, to publication and dissemination of results.
- Embedded patient researchers
-
An embedded researcher works inside a host organization as a member of staff while also maintaining an affiliation with an academic institution. Embedded patient researchers are people with lived experience who carry out research in partnership with health-care teams.
- Evidence-based advocacy
-
The application of scientific principles and evidence to health advocacy.
- Health advocacy
-
Activities aimed at creating positive system change by increasing access to care, navigating the health-care system, mobilizing resources and funding, addressing health inequities and influencing health policy in general.
- Patient advocates
-
People with lived experience as patients or as partners, family members or informal caregivers of a person with a health condition. Patient advocates work for themselves and their patient community to increase awareness, access to resources and help advance research and education.
- Patient engagement
-
Patient engagement encompasses activities such as providing information or disseminating ideas or results to the public and patient communities, which are aimed at increasing awareness of scientific processes. Patient engagement represents a lower level of inclusion in research than patient involvement.
- Patient-led PROs and PROMs
-
Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs) in which patients have provided input to inform the methods and processes used.
- Patient-reported outcome measures
-
(PROMs). Tools or instruments that are used to measure patient-reported outcomes.
- Patient-reported outcomes
-
The FDA defines a patient-reported outcome (PRO) as “a report that comes directly from the patient (i.e. study subject) about the status of a patient’s health condition without amendment or interpretation of the patient’s response by a clinician or anyone else.”
- Preference research
-
Preference research aims at understanding the preferences, needs and expectations of key stakeholders in the health-care system, including patients, caregivers and health-care professionals.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferretti, M.T., Uccheddu, M.B., Flanagan, R. et al. Inclusion in neurological research: empowering people living with neurological diseases. Nat Rev Neurol 21, 159–170 (2025). https://doi.org/10.1038/s41582-024-01047-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-024-01047-6