Abstract
The treatment of Alzheimer disease (AD) has crossed a pivotal threshold, marked by the landmark approvals of the first-ever disease-modifying therapies. These immunotherapies, specifically monoclonal antibodies (mAbs) that target various amyloid-β (Aβ) species including proto-fibrillar and fibrillar forms, substantially lower levels of Aβ in the brain. The therapies have collectively demonstrated the ability to slow cognitive and clinical decline in large placebo-controlled trials, ushering a new era in the management of AD. Here, we review recent progress made in bringing amyloid-lowering mAb therapies to the clinic and explore future directions in this rapidly evolving field. We also delve into the current understanding of AD as a biological continuum, from its asymptomatic preclinical stage to its clinically overt dementia stage. We explore how this conceptualization provides a regulatory framework to evaluate amyloid-lowering mAbs across the entire spectrum of the disease. Additionally, we review key factors that affect the integration of these treatments into clinical practice.
Key points
-
The approval of the first disease-modifying therapies — monoclonal antibodies (mAbs) targeting amyloid-β (Aβ) — marks a pivotal shift in Alzheimer disease (AD) management.
-
The immunotherapies significantly reduce brain Aβ levels and have shown the ability to slow cognitive and clinical decline in large, placebo-controlled trials.
-
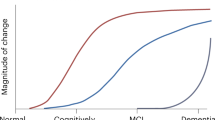
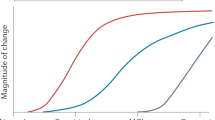
AD is now conceptualized as a biological continuum, from a preclinical asymptomatic stage to full-blown dementia.
-
Whereas some experts question the modest clinical improvements of amyloid-lowering mAbs, newer therapies such as lecanemab and donanemab show more promising results in reducing amyloid plaques and slowing cognitive decline, with manageable risks such as amyloid-related imaging abnormalities.
-
Implementing mAbs in routine care requires early and accurate diagnosis, access to advanced diagnostics (for example, PET, cerebrospinal fluid and blood biomarkers), and infrastructure for infusions, MRI monitoring and long-term follow-up, posing logistical challenges.
-
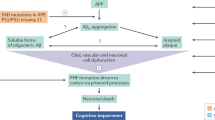
Because Aβ clearance alone slows progression by only ~35%, future strategies must target the disease, as well as other pathologies such as tau, inflammation and vascular issues, earlier. Combination therapies are being explored to address the multifactorial nature of the disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nichols, E. et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7, e105–e125 (2022).
Alzheimer’s Association. Alzheimer’s Disease Facts and Figures (Alzheimer’s Association, 2023).
Hebert, L. E. et al. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 80, 1778–1783 (2013).
Jutkowitz, E. et al. Societal and family lifetime cost of dementia: implications for policy. JAMA Neurol. 77, 889–896 (2020).
Sperling, R. A. et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292 (2011).
Jack, C. R. et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Jack, C. R. Jr et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association workgroup. Alzheimers Dement. 20, 5143–5169 (2024).
Hansson, O. et al. Blood biomarkers for Alzheimer’s disease. Nat. Rev. Neurol. 17, 563–581 (2021).
FDA. FDA clears first blood test used in diagnosing Alzheimer’s disease. FDA https://www.fda.gov/news-events/press-announcements/fda-clears-first-blood-test-used-diagnosing-alzheimers-disease (2025).
FDA. Early Alzheimer’s disease: developing drugs for treatment. FDA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/early-alzheimers-disease-developing-drugs-treatment (2024).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Rafii, M. S. & Aisen, P. S. Detection and treatment of Alzheimer’s disease in its preclinical stage. Nat. Aging 3, 520–531 (2023).
Leisher, S. et al. Amyloid-lowering monoclonal antibodies for the treatment of early Alzheimer’s disease. CNS Drugs 37, 671–677 (2023).
Rabinovici, G. D. & La Joie, R. Amyloid-targeting monoclonal antibodies for Alzheimer disease. JAMA 330, 507–509 (2023).
Sperling, R. A. et al. Trial of solanezumab in preclinical Alzheimer’s disease. N. Engl. J. Med. 389, 1096–1107 (2023).
Ostrowitzki, S. et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch. Neurol. 69, 198–207 (2012).
Bateman, R. J. et al. Two phase 3 trials of gantenerumab in early Alzheimer’s disease. N. Engl. J. Med. 389, 1862–1876 (2023).
Bateman, R. J. et al. Safety and efficacy of long-term gantenerumab treatment in dominantly inherited Alzheimer’s disease: an open-label extension of the phase 2/3 multicentre, randomised, double-blind, placebo-controlled platform DIAN-TU Trial. Lancet Neurol. 24, 316–330 (2025).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016).
Budd Haeberlein, S. et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimers Dis. 9, 197–210 (2022).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Mintun, M. A. et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med. 384, 1691–1704 (2021).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512–527 (2023).
Cummings, J. et al. Lecanemab: appropriate use recommendations. J. Prev. Alzheimers Dis. 10, 362–377 (2023).
Rabinovici, G. D. et al. Donanemab: appropriate use recommendations. J. Prev. Alzheimers Dis. 12, 100150 (2025).
Filippi, M. et al. Amyloid-related imaging abnormalities and β-amyloid-targeting antibodies: a systematic review. JAMA Neurol. 79, 291–304 (2022).
Sperling, R. A. Lecanemab for the treatment of early Alzheimer’s disease: the extension of efficacy results from clarity AD. In The 16th Clinical Trials on Alzheimer’s Disease (CTAD) Conference (CTAD, 2023).
Johnson, K. Biomarker assessments from clarity AD: downstream implications of targeting protofibrils and tau as a predictive biomarker. In The 16th Clinical Trials on Alzheimer’s Disease (CTAD) Conference (CTAD, 2023).
Mintun, M. A. Predicting efficacy in donanemab-treated participants. In The 16th Clinical Trials on Alzheimer’s Disease (CTAD) Conference (CTAD, 2023).
Pugh, M. A. M. TRAILBLAZER-ALZ 3 — a deeper dive into time-to-event trials. Alzheimers Dement. 19, e070865 (2023).
Rafii, M. S. et al. The AHEAD 3-45 Study: design of a prevention trial for Alzheimer’s disease. Alzheimers Dement. 19, 1227–1233 (2023).
Willis, B. A. et al. Model‐based assessment of lecanemab maintenance dosing regimen shows continued suppression of amyloid plaque, disease progression. Alzheimers Dement. 20, e089308 (2025).
Bateman, R. J. et al. The DIAN-TU Next Generation Alzheimer’s prevention trial: adaptive design and disease progression model. Alzheimers Dement. 13, 8–19 (2017).
Grimm, H. P. et al. Delivery of the Brainshuttle™ amyloid-beta antibody fusion trontinemab to non-human primate brain and projected efficacious dose regimens in humans. mAbs 15, 2261509 (2023).
Kulic, L. Trontenimab. In The 17th Clinical Trials on Alzheimer’s Disease (CTAD) Conference (CTAD, 2024).
Phase Ib/IIa Brainshuttle AD study of trontinemab. In AD/PD 2025 International Conference on Alzheimer’s and Parkinson’s Diseases (2025).
Jin, Y. Remternetug: preliminary clinical results. In AD/PD 2023 International Conference on Alzheimer’s and Parkinson’s Diseases (2023).
Alexander, R. Post-FDA approval studies of donanemab. In Alzheimer’s Association International Conference (AAIC) 2024 (AICC, 2024).
Willis, B. A., Penner, N., Rawal, S., Aluri, J. & Reyderman, L. Subcutaneous (SC) lecanemab is predicted to achieve comparable efficacy and improved safety compared to lecanemab IV in early Alzheimer’s disease (AD). Alzheimers Dement. 19, e082852 (2023).
Aisen, P. S. et al. Shifting the focus: amyloid reduction as a surrogate endpoint in Alzheimer’s disease. Alzheimers Dement. 20, 1–15 (2024).
Sperling, R. A. et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s association research roundtable workgroup. Alzheimers Dement. 7, 367–385 (2011).
Salloway, S. et al. Amyloid-related imaging abnormalities in amyloid-modifying therapies. Alzheimers Dement. 18, 1722–1732 (2022).
Yates, P. A. et al. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front. Neurol. 4, 205 (2014).
Barakos, J. et al. Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with anti-amyloid beta therapy. J. Prev. Alzheimers Dis. 9, 211–220 (2022).
Sims, J. Modified titration of donanemab demonstrated reduction of ARIA-E in early symptomatic Alzheimer’s disease patients in phase 3b study. In The 17th Clinical Trials on Alzheimer’s Disease (CTAD) Conference (CTAD, 2024).
Acknowledgements
Funding support includes grant U24AG057437 to P.S.A. and R61AG066543, R33AG066543 and R01AG073979 to M.S.R.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.S.R. is employed by the University of Southern California and the Alzheimer’s Therapeutics Research Institute (ATRI) and has received grants or contracts from Eisai and Eli Lilly, which were paid to his institution. He has received consulting fees from AC Immune and Ionis. He has participated on a data safety monitoring board or an advisory board for Alzheon, Alnylam, Biohaven, Embic, Prescient Imaging, Positrigo and Recall Therapeutics. P.S.A. has research grants from the National Institutes of Health, Eli Lilly and Eisai, and consults with Merck, Roche, BMS, Genentech, Abbvie, Biogen, ImmunoBrain Checkpoint, Arrowhead, AltPep and Neurimmune.
Peer review
Peer review information
Nature Reviews Neurology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Alzheimer’s Network for Treatment and Diagnostics: www.ALZ-NET.org
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rafii, M.S., Aisen, P.S. Amyloid-lowering immunotherapies for Alzheimer disease: current status and future directions. Nat Rev Neurol 21, 490–498 (2025). https://doi.org/10.1038/s41582-025-01123-5
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41582-025-01123-5
This article is cited by
-
Research progress of exosomes used in the Alzheimer's disease treatment
Discover Nano (2025)