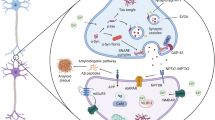
Abstract
Neurodegenerative diseases such as Alzheimer disease (AD), Parkinson disease, frontotemporal lobar degeneration and multiple system atrophy (MSA) are characterized pathologically by deposition of specific proteins in the brain. Five major neurodegenerative disease-associated proteins — amyloid-β (Aβ), tau, α-synuclein, TAR DNA-binding protein 43 (TDP43) and fused in sarcoma (FUS) — are commonly encountered, and the disease specificity and neurotoxicity of the fibrillar protein assemblies are determined by factors such as the protein type, fibril structure, degree of multimerization and post-translational modifications. This article reviews the latest advances in PET technologies aimed at visualizing neurodegenerative proteinopathies, and highlights the importance of these technologies for emerging diagnostic and therapeutic approaches. PET allows Aβ deposition to be visualized throughout the natural history of AD and following anti-Aβ immunotherapies. However, whether this technology can visualize specific Aβ assembly subspecies primarily targeted by the treatment remains inconclusive. Various PET radiotracers can capture AD-type tau deposits, although only a few are known to react with non-AD tau pathologies, and cryo-electron microscopy has revealed the mode of binding of these compounds to different tau protofibrils. High-contrast PET imaging of α-synuclein lesions in MSA is a recent development in the field, and gradual progress is being made towards visualization of other, less abundant α-synuclein pathologies. Imaging of TDP43 and FUS deposits presents particular challenges, which might be overcome by establishing public–private partnerships focused on biomarker development.
Key points
-
Extensive research programmes have aimed to develop small-molecule PET radiotracers for protein aggregates that are implicated in Alzheimer disease (AD) and other neurodegenerative disorders.
-
Amyloid-β (Aβ) PET has been applied in clinical practice, providing robust diagnostic indicators and neuropathology-based outcome measures for trials of anti-Aβ disease-modifying treatments.
-
Diverse tau PET tracers can capture AD-type tau assemblies with varying degrees of off-target binding, whereas only a limited subset can yield high contrast for non-AD-type tau deposits.
-
Cryo-electron microscopy of disease-specific tau fibril folds has identified at least two distinct binding modes of PET radioligands, explaining their selectivity for AD versus non-AD tau pathologies.
-
Despite selectivity issues, high-contrast PET tracers for α-synuclein deposits in multiple system atrophy have become clinically available, and the possibility of visualizing α-synuclein fibrils in Parkinson disease and dementia with Lewy bodies has been demonstrated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Alzheimer, A. Über eine eigenartige Erkrankung der Hirnrinde [German]. Allg. Z. Psychiat. 64, 146–148 (1907).
Kwon, S., Iba, M., Kim, C. & Masliah, E. Immunotherapies for aging-related neurodegenerative diseases — emerging perspectives and new targets. Neurotherapeutics 17, 935–954 (2020).
Villemagne, V. L., Fodero-Tavoletti, M. T., Masters, C. L. & Rowe, C. C. Tau imaging: early progress and future directions. Lancet Neurol. 4, 114–124 (2015).
Wilson et al. Hallmarks of neurodegenerative diseases. Cell 186, 693–714 (2023).
Josephs, K. A. et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 122, 137–153 (2011).
Wong, Y. C. & Krainc, D. α-Synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat. Med. 23, 1–13 (2017).
Neumann, M., Lee, E. B. & Mackenzie, I. R. Frontotemporal lobar degeneration TDP-43-immunoreactive pathological subtypes: clinical and mechanistic significance. Adv. Exp. Med. Biol. 1231, 201–217 (2021).
Harrison, R. S., Sharpe, P. C., Singh, Y. & Fairlie, D. P. Amyloid peptides and proteins in review. Rev. Physiol. Biochem. Pharmacol. 159, 1–77 (2007).
Nordberg, A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol. 3, 519–527 (2004).
Pike, V. PET radiotracers: crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol. Sci. 30, 431–440 (2009).
Scheres, S. H. W., Ryskeldi-Falcon, B. & Goedert, M. Molecular pathology of neurodegenerative diseases by cryo-EM of amyloids. Nature 621, 701–710 (2023).
Chen, X.-Q. & Mobley, W. C. Alzheimer disease pathogenesis: insights from molecular and cellular biology studies of oligomeric Aβ and tau species. Front. Neurosci. 13, 659 (2019).
Sehlin, D. et al. Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer’s disease. Nat. Commun. 7, 10759 (2016).
Wang, J., Dickson, D. W., Trojanowski, J. Q. & Lee, V. M. The levels of soluble versus insoluble brain Aβ distinguish Alzheimer’s disease from normal and pathologic aging. Exp. Neurol. 158, 328–337 (1999).
Tenreiro, S., Eckermann, K. & Outeiro, T. F. Protein phosphorylation in neurodegeneration: friend or foe? Front. Mol. Neurosci. 7, 42 (2014).
Cummings, J. The role of biomarkers in Alzheimer’s disease drug development. Adv. Exp. Med. Biol. 1118, 29–61 (2019).
Jack, C. R. Jr. et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s association workgroup. Alzheimers Dement. 20, 5143–5169 (2024).
Jack, C. R. Jr. et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87, 539–547 (2016).
Simuni, T. et al. A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research. Lancet Neurol. 23, 178–190 (2024).
Höglinger, G. U. et al. A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria. Lancet Neurol. 23, 191–204 (2024).
Boccardi, M. et al. The Strategic Biomarker Roadmap for the validation of Alzheimer’s diagnostic biomarkers: methodological update. Eur. J. Nucl. Med. Mol. Imaging 48, 2070–2085 (2021).
Chiotis, K. et al. Clinical validity of increased cortical uptake of amyloid ligands on PET as a biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol. Aging 52, 214–227 (2017).
Wolters, E. E. et al. Clinical validity of increased cortical uptake of [18F]flortaucipir on PET as a biomarker for Alzheimer’s disease in the context of a structured 5-phase biomarker development framework. Eur. J. Nucl. Med. Mol. Imaging 48, 2097–2109 (2021).
Klunk, W. E. et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann. Neurol. 55, 306–319 (2004).
Doraiswamy, P. M. et al. Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol. Psychiatry 19, 1044–10451 (2014).
Shchebinin, S. et al. Association of amyloid reduction after donanemab treatment with tau pathology and clinical outcomes. JAMA Neurol. 79, 1015–1024 (2022).
Pemberton, H. G. et al. Quantification of amyloid PET for future clinical use: a state-of-the-art review. Eur. J. Nucl. Med. Mol. Imaging 49, 3508–3528 (2022).
Klunk, W. E. et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 11, 1–15 (2015).
Clark, C. M. et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 11, 669–678 (2012).
Curtis, C. et al. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 72, 287–294 (2015).
Sabri, O. et al. Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement. 11, 964–974 (2015).
La Joie, R. et al. Multisite study of the relationships between antemortem [11C]PIB-PET centiloid values and postmortem measures of Alzheimer’s disease neuropathology. Alzheimers Dement. 15, 205–216 (2019).
Yamaguchi, H. et al. Alzheimer type dementia: diffuse type of senile plaques demonstrated by beta protein immunostaining. Prog. Clin. Biol. Res. 317, 467–474 (1989).
Sheng, J. G., Mrak, R. E. & Griffin, W. S. Neuritic plaque evolution in Alzheimer’s disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol. 94, 1–5 (1997).
Mathur, R. et al. A reduced astrocyte response to β-amyloid plaques in the ageing brain associates with cognitive impairment. PLoS ONE 10, e0118463 (2015).
Malek-Ahmadi, M., Perez, S. E., Chen, K. & Mufson, E. J. Neuritic and diffuse plaque associations with memory in non-cognitively impaired elderly. J. Alzheimers Dis. 53, 1641–1652 (2016).
Jack, C. R. Jr et al. An operational approach to National Institute on Aging–Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann. Neurol. 71, 765–775 (2012).
Jansen, W. J. et al. Prevalence of cerebral amyloid pathology in persons without dementia. A meta-analysis. JAMA 313, 1924–1938 (2015).
Petersen, R. C. et al. Mild cognitive impairment due to Alzheimer’s disease in the community. Ann. Neurol. 74, 199–208 (2013).
Donohue, M. C. et al. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 317, 2305–2316 (2017).
Brookmeyer, R. & Abdalla, N. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimers Dement. 14, 981–988 (2018).
Jansen, W. J. et al. Prevalence estimates of amyloid abnormality across the Alzheimer disease clinical spectrum. JAMA Neurol. 79, 228–243 (2022).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Ossenkoppele, R. et al. Prevalence of amyloid PET positivity in dementia syndromes. JAMA 313, 1939–1949 (2015).
Johnson, K. A. et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 9, e1–e16 (2013).
Rabinovici, G. D. et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA 321, 1286–1294 (2019).
de Wilde, A. et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project. JAMA Neurol. 75, 1062–1070 (2018).
Altomare, D. et al. Clinical effect of early vs late amyloid positron emission tomography in memory clinic patients: the AMYPAD-DPMS randomized clinical trial. JAMA Neurol. 80, 548–557 (2023).
van Maurik, I. S. et al. A more precise diagnosis by means of amyloid PET contributes to delayed institutionalization, lower mortality, and reduced care costs in a tertiary memory clinic setting. Alzheimers Dement. 19, 2006–2013 (2022).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016).
Mintun, M. A. et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med. 384, 1691–1704 (2021).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2022).
Salloway, S. et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat. Med. 27, 1187–1196 (2021).
Reardon, S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature 613, 227–228 (2023).
Klunk, W. E. et al. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-β in Alzheimer’s disease brain but not in transgenic mouse brain. J. Neurosci. 25, 10598–10606 (2005).
Maeda, J. et al. Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer’s disease enabled by positron emission tomography. J. Neurosci. 27, 10957–10968 (2007).
Saido, T. C. et al. Dominant and differential deposition of distinct β-amyloid peptide species, AβN3(pE), in senile plaques. Neuron 14, 457–466 (1995).
Kawarabayashi, T. et al. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J. Neurosci. 21, 372–381 (2001).
Bayer, T. A. & Wirths, O. Focusing the amyloid cascade hypothesis on N-truncated Abeta peptides as drug targets against Alzheimer’s disease. Acta Neuropathol. 127, 787–801 (2014).
Thal, D. R., Walter, J., Saido, T. C. & Fändrich, M. Different aspects of Alzheimer’s disease-related amyloid β-peptide pathology and their relationship to amyloid positron emission tomography imaging and dementia. Acta Neuropathol. Commun. 7, 178 (2019).
Bao, F. et al. Different β-amyloid oligomer assemblies in Alzheimer brains correlate with age of disease onset and impaired cholinergic activity. Neurobiol. Aging 33, 825.e1–825.e13 (2012).
van Berckel, B. N. M. et al. Longitudinal amyloid imaging using 11C-PiB: methodologic considerations. J. Nucl. Med. 54, 1570–1576 (2013).
Lowe, V. J. et al. White matter reference region in PET studies of 11C-Pittsburgh compound B uptake: effects of age and amyloid-β deposition. J. Nucl. Med. 59, 1583–1589 (2018).
Southekal, S. et al. Flortaucipir F 18 quantitation using parametric estimation of reference signal intensity. J. Nucl. Med. 59, 944–951 (2018).
Okamura, N. et al. Quinoline and benzimidazole derivatives: candidate probes for in vivo imaging of tau pathology in Alzheimer’s disease. J. Neurosci. 25, 10857–10862 (2005).
Chien, D. T. et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J. Alzheimers Dis. 34, 457–468 (2013).
Chien, D. T. et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J. Alzheimers Dis. 38, 171–184 (2014).
Maruyama, M. et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 79, 1094–1108 (2013).
Johnson, K. A. et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 79, 110–119 (2016).
Harada, R. et al. 18F-THK5351: a novel PET radiotracer for imaging neurofibrillary pathology in Alzheimer disease. J. Nucl. Med. 57, 208–214 (2016).
Ng, K. P. et al. Monoamine oxidase B inhibitor, selegiline, reduces 18F-THK5351 uptake in the human brain. Alzheimers Res. Ther. 9, 25 (2017).
Marquié, M. et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann. Neurol. 78, 787–800 (2015).
Ni, R. et al. Comparative in vitro and in vivo quantifications of pathologic tau deposits and their association with neurodegeneration in tauopathy mouse models. J. Nucl. Med. 59, 960–966 (2018).
Hashimoto, H. et al. Identification of a major radiometabolite of [11C]PBB3. Nucl. Med. Biol. 42, 905–910 (2015).
Ikonomovic, M. D., Abrahamson, E. E., Price, J. C., Mathis, C. A. & Klunk, W. E. [F-18]AV-1451 positron emission tomography retention in choroid plexus: more than “off-target” binding. Ann. Neurol. 80, 307–308 (2016).
Aguero, C. et al. Autoradiography validation of novel tau PET tracer [F-18]-MK-6240 on human postmortem brain tissue. Acta Neuropathol. Commun. 7, 37 (2019).
Honer, M. et al. Preclinical evaluation of 18F-RO6958948, 11C-RO6931643, and 11C-RO6924963 as novel PET radiotracers for imaging tau aggregates in Alzheimer disease. J. Nucl. Med. 59, 675–681 (2018).
Kroth, H. et al. Discovery and preclinical characterization of [18F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur. J. Nucl. Med. Mol. Imaging 46, 2178–2189 (2019).
Sanabria Bohórquez, S. et al. [18F]GTP1 (Genentech Tau Probe 1), a radioligand for detecting neurofibrillary tangle tau pathology in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 46, 2077–2089 (2019).
Tagai, K. et al. High-contrast in vivo imaging of tau pathologies in Alzheimer’s and non-Alzheimer’s disease tauopathies. Neuron 109, 42–58.e8 (2021).
Baker, S. L. et al. Evaluation of [18F]-JNJ-64326067-AAA tau PET tracer in humans. J. Cereb. Blood Flow. Metab. 41, 3302–3313 (2021).
Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D. & Crowther, R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3, 519–526 (1989).
Wang, Y. & Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 17, 5–21 (2016).
Dickson, D. W., Kouri, N., Murray, M. E. & Josephs, K. A. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J. Mol. Neurosci. 45, 384–389 (2011).
Tarutani, A. et al. Ultrastructural and biochemical classification of pathogenic tau, α-synuclein and TDP-43. Acta Neuropathol. 143, 613–640 (2022).
Leuzy, A. et al. Tau PET imaging in neurodegenerative tauopathies — still a challenge. Mol. Psychiatry 24, 1112–1134 (2019).
Marquié, M. et al. Pathological correlations of [F-18]-AV-1451 imaging in non-Alzheimer tauopathies. Ann. Neurol. 81, 117–128 (2017).
Ono, M. et al. Distinct binding of PET ligands PBB3 and AV-1451 to tau fibril strains in neurodegenerative tauopathies. Brain 140, 764–780 (2017).
Malarte, M. L. et al. Discriminative binding of tau PET tracers PI2620, MK6240 and RO948 in Alzheimer’s disease, corticobasal degeneration and progressive supranuclear palsy brains. Mol. Psychiatry 28, 1272–1283 (2023).
Brendel, M. et al. Assessment of 18F-PI-2620 as a biomarker in progressive supranuclear palsy. JAMA Neurol. 77, 1408–1419 (2020).
Endo, H. et al. In vivo binding of a tau imaging probe, [11C]PBB3, in patients with progressive supranuclear palsy. Mov. Disord. 34, 744–754 (2019).
Nakano, Y. et al. PET-based classification of corticobasal syndrome. Parkinsonism Relat. Disord. 98, 92–98 (2022).
Li, L. et al. Clinical utility of 18F-APN-1607 tau PET imaging in patients with progressive supranuclear palsy. Mov. Disord. 36, 2314–2323 (2021).
Endo, H. et al. A machine learning-based approach to discrimination of tauopathies using [18F]PM-PBB3 PET images. Mov. Disord. 37, 2236–2246 (2022).
Tezuka, T. et al. Evaluation of [18F]PI-2620, a second-generation selective tau tracer, for assessing four-repeat tauopathies. Brain Commun. 3, fcab190 (2021).
Shi, Y. et al. Structure-based classification of tauopathies. Nature 598, 359–363 (2021).
Shi, Y. et al. Cryo-EM structures of tau filaments from Alzheimer’s disease with PET ligand APN-1607. Acta Neuropathol. 141, 697–708 (2021).
Alosco, M. L. et al. 18F-MK-6240 tau PET as a biomarker for chronic traumatic encephalopathy: case series of 10 symptomatic former National Football League players. Alzheimers Dement. 18, e066995 (2022).
Merz, G. E. et al. Stacked binding of a PET ligand to Alzheimer’s tau paired helical filaments. Nat. Commun. 14, 3048 (2023).
Kunach, P. et al. Cryo-EM structure of Alzheimer’s disease tau filaments with PET ligand MK-6240. Nat. Commun. 15, 8497 (2023).
Alosco, M. L. et al. Associations between near end-of-life flortaucipir PET and postmortem CTE-related tau neuropathology in six former American football players. Eur. J. Nucl. Med. Mol. Imaging 50, 435–452 (2023).
Takahata, K. et al. Grouping tau topologies in former boxers assessed by PET with 18F-PM-PBB3: a reappraisal of dementia pugilistica. Alzheimers Dement. 16, e041510 (2020).
Qi, C. et al. Identical tau filaments in subacute sclerosing panencephalitis and chronic traumatic encephalopathy. Acta Neuropathol. Commun. 11, 74 (2023).
Qi, C. et al. Tau filaments from amyotrophic lateral sclerosis/parkinsonism-dementia complex adopt the CTE fold. Proc. Natl Acad. Sci. USA 120, e2306767120 (2023).
Braak, H., Alafuzoff, I., Arzberger, T., Kretzschmar, H. & Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389–404 (2006).
Braak, H. & Braak, E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging 16, 271–278 (1995).
Schöll, M. et al. PET imaging of tau deposition in the aging human brain. Neuron 89, 971–982 (2016).
Pascoal, T. A. et al. 18F-MK-6240 PET for early and late detection of neurofibrillary tangles. Brain 143, 2818–2830 (2020).
Leuzy, A. et al. Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of Alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 77, 955–965 (2020).
Ossenkoppele, R. et al. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA 320, 1151–1162 (2018).
Jie, C. V. M. L., Treyer, V., Schibli, R. & Mu, L. Tauvid™: the first FDA-approved PET tracer for imaging tau pathology in Alzheimer’s disease. Pharmaceuticals 14, 110 (2021).
Leuzy, A. et al. Comparison of group-level and individualized brain regions for measuring change in longitudinal tau positron emission tomography in Alzheimer disease. JAMA Neurol. 80, 614–623 (2023).
Vogel, J. W. et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 27, 871–881 (2021).
Villemagne, V. L. et al. CenTauR: toward a universal scale and masks for standardizing tau imaging studies. Alzheimers Dement. 15, e12454 (2023).
Fleisher, A. S. et al. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 77, 829–839 (2020).
Groot, C. et al. Tau positron emission tomography for predicting dementia in individuals with mild cognitive impairment. JAMA Neurol. 81, 845–856 (2024).
Chen, S.-D. et al. Staging tau pathology with tau PET in Alzheimer’s disease: a longitudinal study. Transl. Psychiatry 11, 483 (2021).
Crary, J. F. et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 128, 755–766 (2014).
Wuestefeld, A. et al. Age-related and amyloid-beta-independent tau deposition and its downstream effects. Brain 146, 3192–3205 (2023).
Villemagne, V. L. et al. What is T+? A Gordian Knot of tracers, thresholds, and topographies. J. Nucl. Med. 62, 614–619 (2021).
Ossenkoppele, R. et al. Accuracy of tau positron emission tomography as a prognostic marker in preclinical and prodromal Alzheimer disease: a head-to-head comparison against amyloid positron emission tomography and magnetic resonance imaging. JAMA Neurol. 78, 961–971 (2021).
Altomare, D. et al. Diagnostic value of amyloid-PET and tau-PET: a head-to-head comparison. Eur. J. Nucl. Med. Mol. Imaging 48, 2200–2211 (2021).
Shimohama, S. et al. Impact of amyloid and tau PET on changes in diagnosis and patient management. Neurology 100, e264–e274 (2023).
Smith, R. et al. Clinical utility of tau positron emission tomography in the diagnostic workup of patients with cognitive symptoms. JAMA Neurol. 80, 749–756 (2023).
Budd Haeberlein, S. et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimers Dis. 9, 197–210 (2022).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512–527 (2023).
Teng, E. et al. Safety and efficacy of semorinemab in individuals with prodromal to mild Alzheimer disease: a randomized clinical trial. JAMA Neurol. 79, 758–767 (2022).
Edwards, A. L. et al. Exploratory tau biomarker results from a multiple ascending-dose study of BIIB080 in Alzheimer disease: a randomized clinical trial. JAMA Neurol. 80, 1344–1352 (2023).
Mummery, C. J. et al. Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer’s disease: a phase 1b, randomized, placebo-controlled trial. Nat. Med. 29, 1437–1447 (2023).
Ziogas, N. et al. Exploratory clinical outcomes from BIIB080 (MAPT ASO) phase 1b multiple ascending dose and long-term extension study in mild Alzheimer’s disease. J. Prev. Alz. Dis. 10, S41 (2023).
Endo, H. et al. Correlation of 18F-PM-PBB3 (18F-florzolotau) tau PET imaging with postmortem neuropathological findings in a case with progressive supranuclear palsy (P9-11.015) [abstract]. Neurology https://doi.org/10.1212/WNL.0000000000201875 (2023).
Tagai, K. et al. An optimized reference tissue method for quantification of tau protein depositions in diverse neurodegenerative disorders by PET with 18F-PM-PBB3 (18F-APN-1607). Neuroimage 264, 119763 (2022).
Leuzy, A. et al. Harmonizing tau positron emission tomography in Alzheimer’s disease: the CenTauR scale and the joint propagation model. Alzheimers Dement. 20, 5833–5848 (2024).
van Eimeren, T. et al. Neuroimaging biomarkers for clinical trials in atypical parkinsonian disorders: proposal for a neuroimaging biomarker utility system. Alzheimers Dement. 11, 301–309 (2019).
Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003).
Cykowski, M. D. et al. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138, 2293–2309 (2015).
Kikuchi, A. et al. In vivo visualization of α-synuclein deposition by carbon-11-labelled 2-[2-(2-dimethylaminothiazol-5-yl)ethenyl]-6-[2-(fluoro)ethoxy]benzoxazole positron emission tomography in multiple system atrophy. Brain 133, 1772–1778 (2010).
Verdurand, M. et al. Amyloid-beta radiotracer [18F]BF-227 does not bind to cytoplasmic glial inclusions of postmortem multiple system atrophy brain tissue. Contrast Media Mol. Imaging 2018, 9165458 (2018).
Koga, S., Ono, M., Sahara, N., Higuchi, M. & Dickson, D. W. Fluorescence and autoradiographic evaluation of tau PET ligand PBB3 to α-synuclein pathology. Mov. Disord. 32, 884–892 (2017).
Liu, F. T. et al. 18F-florzolotau tau positron emission tomography imaging in patients with multiple system atrophy-parkinsonian subtype. Mov. Disord. 37, 1915–1923 (2022).
Roshanbin, S. et al. In vivo imaging of α-synuclein with antibody-based PET. Neuropharmacology 208, 108985 (2022).
Pees, J. Y. et al. Development of pyridothiophene compounds for PET imaging of α-synuclein aggregates. Chem. Eur. J. 30, e202303921 (2024).
Kim, H. Y. et al. A novel brain PET radiotracer for imaging alpha synuclein fibrils in multiple system atrophy. J. Med. Chem. 66, 12185–12202 (2023).
Zeng, Q. et al. Discovery and evaluation of imidazo[2,1-b][1,3,4]thiadiazole derivatives as new candidates for α-synuclein PET imaging. J. Med. Chem. 67, 12345–12358 (2024).
Zhang, X. et al. Radiosynthesis and in vivo evaluation of two PET radioligands for imaging α-synuclein. Appl. Sci. 4, 66–78 (2014).
Kuebler, L. et al. [11C]MODAG-001 — towards a PET tracer targeting α-synuclein aggregates. Eur. J. Nucl. Med. Mol. Imaging 48, 1759–1772 (2021).
Korat, Š. et al. Toward novel [18F]fluorine-labeled radiotracers for the imaging of α-synuclein fibrils. Front. Aging Neurosci. 14, 830704 (2022).
Bonanno, F. et al. Advancing Parkinson’s disease diagnostics: the potential of arylpyrazolethiazole derivatives for imaging α-synuclein aggregates. ACS Omega 9, 24774–24788 (2024).
Maurer, A. et al. 11C radiolabeling of anle253b: a putative PET tracer for Parkinson’s disease that binds to α-synuclein fibrils in vitro and crosses the blood-brain barrier. ChemMedChem 15, 411–415 (2020).
Janssen, B. et al. Identification of a putative α-synuclein radioligand using an in silico similarity search. Mol. Imaging Biol. 25, 704–719 (2023).
Di Nanni, A. et al. A fluorescent probe as a lead compound for a selective α-synuclein PET tracer. ACS Omega 8, 24339–24352 (2023).
Wu, J. et al. Development of an ¹⁸F-labeled azobenzothiazole tracer for α-synuclein aggregates in the brain. Org. Biomol. Chem. 22, 3724–3732 (2024).
Antonschmidt, L. et al. The clinical drug candidate anle138b binds in a cavity of lipidic α-synuclein fibrils. Nat. Commun. 13, 5285 (2022).
Hsieh, C. J. et al. Alpha synuclein fibrils contain multiple binding sites for small molecules. ACS Chem. Neurosci. 9, 2521–2527 (2018).
Endo, H. et al. Imaging α-synuclein pathologies in animal models and patients with Parkinson’s and related diseases. Neuron 112, 2540–2557.e8 (2024).
Fitzpatrick, A. W. P. et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547, 185–190 (2017).
Spillantini, M. G., Crowther, R. A., Jakes, R., Hasegawa, M. & Goedert, M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl Acad. Sci. USA 95, 6469–6473 (1998).
Macdonald, J. A. et al. Assembly of α-synuclein and neurodegeneration in the central nervous system of heterozygous M83 mice following the peripheral administration of α-synuclein seeds. Acta Neuropathol. Commun. 9, 189 (2021).
Miranda-Azpiazu, P. et al. Identification and in vitro characterization of C05-01, a PBB3 derivative with improved affinity for alpha-synuclein. Brain Res. 1749, 147131 (2020).
Matsuoka, K. et al. High-contrast imaging of α-synuclein pathologies in living patients with multiple system atrophy. Mov. Disord. 37, 2159–2161 (2022).
Smith, R. et al. The α-synuclein PET tracer [18F] ACI-12589 distinguishes multiple system atrophy from other neurodegenerative diseases. Nat. Commun. 14, 6750 2023).
Xiang, J. et al. Development of an α-synuclein positron emission tomography tracer for imaging synucleinopathies. Cell 186, 3350–3367.e19 (2023).
Arseni, D. et al. Structure of pathological TDP-43 filaments from ALS with FTLD. Nature 601, 139–143 (2022).
Knight, A. C. et al. Head-to-head comparison of tau-PET radioligands for imaging TDP-43 in post-mortem ALS brain. Mol. Imaging Biol. 25, 513–527 (2023).
Arseni, D. et al. TDP-43 forms amyloid filaments with a distinct fold in type A FTLD-TDP. Nature 620, 898–903 (2023).
Arseni, D. et al. Heteromeric amyloid filaments of ANXA11 and TDP-43 in FTLD-TDP type C. Nature 634, 662–668 (2024).
Bigio, E. H. et al. Inclusions in frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP) and amyotrophic lateral sclerosis (ALS), but not FTLD with FUS proteinopathy (FTLD-FUS), have properties of amyloid. Acta Neuropathol. 125, 463–465 (2013).
Seredenina, T. et al. Discovery and optimization of the first-in-class TDP-43 PET tracer. Alzheimers Dement. 19, e075525 (2023).
Spina, S. et al. Comorbid neuropathological diagnoses in early versus late-onset Alzheimer’s disease. Brain 144, 2186–2198 (2021).
Schweighauser, M. et al. Age-dependent formation of TMEM106B amyloid filaments in human brains. Nature 605, 310–314 (2022).
Jiang, Y. X. et al. Amyloid fibrils in FTLD-TDP are composed of TMEM106B and not TDP-43. Nature 605, 304–309 (2022).
Perneel, J. et al. Accumulation of TMEM106B C-terminal fragments in neurodegenerative disease and aging. Acta Neuropathol. 145, 285–302 (2023).
Hampel, H. et al. Blood-based biomarkers for Alzheimer’s disease: mapping the road to the clinic. Nat. Rev. Neurol. 14, 639–652 (2018).
Karikari, T. K. et al. Blood phospho-tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat. Rev. Neurol. 18, 400–418 (2022).
Milà-Alomà, M. et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat. Med. 28, 1797–1801 (2022).
Tagai, K. et al. A novel plasma p-tau181 assay as a specific biomarker of tau pathology in Alzheimer’s disease. Transl. Neurodegener. 13, 44 (2023).
Horie, K. et al. Plasma MTBR-tau243 biomarker identifies tau tangle pathology in Alzheimer’s disease. Nat. Med. 31, 2044–2053 (2025).
Altomare, D. et al. Plasma biomarkers for Alzheimer’s disease: a field-test in a memory clinic. J. Neurol. Neurosurg. Psychiatry 94, 420–427 (2023).
Brum, W. S. et al. A blood-based biomarker workflow for optimal tau-PET referral in memory clinic settings. Nat. Commun. 15, 2311 (2024).
Eberling, J. L., Dave, K. D. & Frasier, M. A. α-Synuclein imaging: a critical need for Parkinson’s disease research. J. Parkinsons Dis. 3, 565–567 (2013).
Suhara, T. et al. Strategies for utilizing neuroimaging biomarkers in CNS drug discovery and development: CINP/JSNP working group report. Int. J. Neuropsychopharmacol. 20, 285–294 (2017).
Thal, D. R. et al. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer’s disease. Acta Neuropathol. 129, 167–182 (2015).
Schöll, M. et al. Biomarkers for tau pathology. Mol. Cell Neurosci. 97, 18–33 (2019).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and reviewed and/or edited the manuscript before submission. M.H, K. Takahata. and H.E. contributed substantially to discussion of the content. M.H., K. Tagai. and H.E. wrote the article.
Corresponding author
Ethics declarations
Competing interests
M.H. holds patents on PBB3, florzolotau and related compounds (JP 5422782/EP 12 884 742.3/CA2894994/HK1208672) and α-synuclein ligands (JP7460176). M.H. is supported by research grants from the Japan Agency for Medical Research and Development (JP24zf0127012 and JP24wm0625001) and Japan Science and Technology Agency (JPMJMS2024). The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Higuchi, M., Tagai, K., Takahata, K. et al. Advances in PET imaging of protein aggregates associated with neurodegenerative disease. Nat Rev Neurol 21, 506–522 (2025). https://doi.org/10.1038/s41582-025-01126-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-025-01126-2