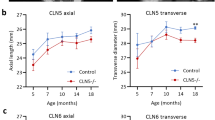
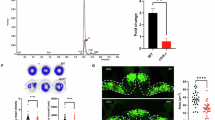
Abstract
The neuronal ceroid lipofuscinoses (NCLs), more commonly known as Batten disease, are a group of fatal inherited neurodegenerative lysosomal storage disorders. Each form is caused by mutations in a different gene, resulting in lysosomal dysfunction, which, by largely unknown mechanisms, has a devastating impact on the central nervous system. The NCLs are grouped together owing to their broadly shared clinical presentations and the presence of autofluorescent storage material. Nevertheless, being caused by deficiencies in dissimilar proteins, marked differences are apparent between NCLs in their clinical presentation and pathology. The effects of disease are not confined to neurons and appear unrelated to autofluorescent storage material, with glial cells also affected. The rest of the body is also affected, with life-limiting disease in the bowel and effects on other body systems, which will also require treatment for maximal therapeutic benefit. Since the development of enzyme replacement therapy for CLN2 disease, much has been learnt about the practicalities of its delivery. Considerable progress has also been made in the understanding of NCL cell biology, disease pathogenesis and potential links to other disorders. Here, we highlight these advances and how they inform the ongoing development of therapeutic strategies and their future prospects.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kohlschütter, A., Schulz, A., Bartsch, U. & Storch, S. Current and emerging treatment strategies for neuronal ceroid lipofuscinoses. CNS Drugs 33, 315–325 (2019).
Mole, S. E. et al. Clinical challenges and future therapeutic approaches for neuronal ceroid lipofuscinosis. Lancet Neurol. 18, 107–116 (2019).
Johnson, T. B. et al. Therapeutic landscape for Batten disease: current treatments and future prospects. Nat. Rev. Neurol. 15, 161–178 (2019).
Augustine, E. F. et al. Management of CLN1 disease: International clinical consensus. Pediatr. Neurol. 120, 38–51 (2021).
Mole, S. E. et al. Guidelines on the diagnosis, clinical assessments, treatment and management for CLN2 disease patients. Orphanet J. Rare Dis. 16, 185 (2021).
Ostergaard, J. R. Juvenile neuronal ceroid lipofuscinosis (Batten disease): current insights. Degener. Neurol. Neuromuscul. Dis. 6, 73–83 (2016).
Anderson, G. W., Goebel, H. H. & Simonati, A. Human pathology in NCL. Biochim. Biophys. Acta 1832, 1807–1826 (2013).
Tyynelä, J., Cooper, J. D., Khan, M. N., Shemilt, S. J. & Haltia, M. Hippocampal pathology in the human neuronal ceroid-lipofuscinoses: distinct patterns of storage deposition, neurodegeneration and glial activation. Brain Pathol. 14, 349–357 (2004).
Hachiya, Y. et al. Mechanisms of neurodegeneration in neuronal ceroid-lipofuscinoses. Acta Neuropathol. 111, 168–177 (2006).
Radke, J., Stenzel, W. & Goebel, H. H. Human NCL neuropathology. Biochim. Biophys. Acta 1852, 2262–2266 (2015).
Markham, A. Cerliponase alfa: first global approval. Drugs 77, 1247–1249 (2017).
Schulz, A. et al. CLN2 study group. Study of intraventricular cerliponase alfa for CLN2 disease. N. Engl. J. Med. 378, 1898–1907 (2018).
Batten, F. E. Cerebral degeneration with symmetrical changes in the maculae in two members of a family. Trans. Ophthalmol. Soc. UK 23, 386–390 (1903).
Stengel, O. C. Beretning om et maerkeligt Sygdomstilfaelde hos fire Sødskende I Nærheden af Röraas. Eyr 1, 347–352 (1826).
Brean, A. An account of a strange instance of disease-Stengel-Batten-Spielmayer-Vogt disease. Tidsskr. Nor. Laegeforen. 124, 970–971 (2004).
Haltia, M. & Goebel, H. H. The neuronal ceroid-lipofuscinoses: a historical introduction. Biochim. Biophys. Acta 1832, 1795–17800 (2013).
Williams, R. E. & Mole, S. E. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology 79, 183–191 (2012).
Zeman, W. & Dyken, P. Neuronal ceroid-lipofuscinosis (Batten’s disease): relationship to amaurotic family idiocy? Pediatrics 44, 570–583 (1969).
Seehafer, S. S. & Pearce, D. A. You say lipofuscin, we say ceroid: defining autofluorescent storage material. Neurobiol. Aging 27, 576–588 (2006).
Vesa, J. et al. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature 376, 584–587 (1995).
Sleat, D. E. et al. Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis. Science 277, 1802–1805 (1997).
Lerner, T. J. et al. Isolation of a novel gene underlying Batten disease, CLN3. Cell 82, 949–957 (1995).
Schulz, A. et al. Impaired cell adhesion and apoptosis in a novel CLN9 Batten disease variant. Ann. Neurol. 56, 342–350 (2004).
Savukoski, M. et al. CLN5, a novel gene encoding a putative transmembrane protein mutated in Finnish variant late infantile neuronal ceroid lipofuscinosis. Nat. Genet. 19, 286–288 (1998).
Wheeler, R. B. et al. The gene mutated in variant late-infantile neuronal ceroid lipofuscinosis (CLN6) and in nclf mutant mice encodes a novel predicted transmembrane protein. Am. J. Hum. Genet. 70, 537–542 (2002).
Kousi, M. et al. Mutations in CLN7/MFSD8 are a common cause of variant late-infantile neuronal ceroid lipofuscinosis. Brain 132, 810–819 (2009).
Ranta, S. et al. The neuronal ceroid lipofuscinoses in human EPMR and mnd mutant mice are associated with mutations in CLN8. Nat. Genet. 23, 233–236 (1999).
Siintola, E. et al. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain 129, 1438–1445 (2006).
Canafoglia, L. et al. Recurrent generalized seizures, visual loss, and palinopsia as phenotypic features of neuronal ceroid lipofuscinosis due to progranulin gene mutation. Epilepsia 55, e56–e59 (2014).
Bras, J., Verloes, A., Schneider, S. A., Mole, S. E. & Guerreiro, R. J. Mutation of the parkinsonism gene ATP13A2 causes neuronal ceroid-lipofuscinosis. Hum. Mol. Genet. 21, 2646–2650 (2012).
Smith, K. R. et al. Cathepsin F mutations cause Type B Kufs disease, an adult-onset neuronal ceroid lipofuscinosis. Hum. Mol. Genet. 22, 1417–1423 (2013).
Staropoli, J. F. et al. A homozygous mutation in KCTD7 links neuronal ceroid lipofuscinosis to the ubiquitin-proteasome system. Am. J. Hum. Genet. 91, 202–208 (2012).
Neufeld, E. F. & Muenzer, J. In: The Online Metabolic and Molecular Bases of Inherited Disease (ed. Valle, D. L. et al.) (McGraw Hill, 2019).
Platt, F. M., Azzo, A., Davidson, B. L., Neufeld, E. F. & Tifft, C. J. Lysosomal storage diseases. Nat. Rev. Dis. Prim. 4, 27 (2018).
Muenzer, J. Overview of the mucopolysaccharidoses. Rheumatol 50, v4–v12 (2011).
McBride, K. L. & Flanigan, K. M. Update in the mucopolysaccharidoses. Semin. Pediatr. Neurol. 37, 100874 (2021).
di Ronza, A. et al. CLN8 is an endoplasmic reticulum cargo receptor that regulates lysosome biogenesis. Nat. Cell Biol. 20, 1370–1377 (2018).
Bajaj, L. et al. A CLN6-CLN8 complex recruits lysosomal enzymes at the ER for Golgi transfer. J. Clin. Invest. 130, 4118–4132 (2020).
Parenti, G., Medina, D. L. & Ballabio, A. The rapidly evolving view of lysosomal storage diseases. EMBO Mol. Med. 13, e12836 (2021).
Nosková, L. et al. Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. Am. J. Hum. Genet. 89, 241–252 (2011).
Benitez, B. A. et al. Exome-sequencing confirms DNAJC5 mutations as cause of adult neuronal ceroid-lipofuscinosis. PLoS One 6, e26741 (2011).
Sands, M. S. & Davidson, B. L. Gene therapy for lysosomal storage diseases. Mol. Ther. 13, 839–849 (2006).
Neufeld, E. F. & Fratantoni, J. C. Inborn errors of mucopolysaccharide metabolism. Science 169, 141–146 (1970).
Kornfeld, S. Lysosomal enzyme targeting. Biochem. Soc. Trans. 18, 367–374 (1990).
Kornfeld, S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 61, 307–330 (1992).
Braulke, T. & Bonifacino, J. S. Sorting of lysosomal proteins. Biochim. Biophys. Acta 1793, 605–614 (2009).
Chang, M. et al. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol. Ther. 16, 649–656 (2008).
Xu, S. et al. Large-volume intrathecal enzyme delivery increases survival of a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol. Ther. 19, 1842–1848 (2011).
Wiseman, J. A. et al. Chronic enzyme replacement to the brain of a late infantile neuronal ceroid lipofuscinosis mouse has differential effects on phenotypes of disease. Mol. Ther. Methods Clin. Dev. 4, 204–212 (2017).
Katz, M. L. et al. Enzyme replacement therapy attenuates disease progression in a canine model of late-infantile neuronal ceroid lipofuscinosis (CLN2 disease). J. Neurosci. Res. 92, 1591–1598 (2014).
Vuillemenot, B. R. et al. Nonclinical evaluation of CNS-administered TPP1 enzyme replacement in canine CLN2 neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 114, 281–293 (2015).
Hu, J. et al. Intravenous high-dose enzyme replacement therapy with recombinant palmitoyl-protein thioesterase reduces visceral lysosomal storage and modestly prolongs survival in a preclinical mouse model of infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 107, 213–221 (2012).
Lu, J. Y. et al. Intrathecal enzyme replacement therapy improves motor function and survival in a preclinical mouse model of infantile neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 116, 98–105 (2015).
Nelvagal, H. R. et al. Cross-species efficacy of enzyme replacement therapy for CLN1 disease in mice and sheep. J. Clin. Invest. 132, e163107 (2022).
Griffey, M. A. et al. Adeno-associated virus 2-mediated gene therapy decreases autofluorescent storage material and increases brain mass in a murine model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 16, 360–369 (2004).
Griffey, M., Macauley, S. L., Ogilvie, J. M. & Sands, M. S. AAV2-mediated ocular gene therapy for infantile neuronal ceroid lipofuscinosis. Mol. Ther. 12, 413–421 (2005).
Griffey, M. A. et al. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol. Ther. 13, 538–547 (2006).
Macauley, S. L. et al. Synergistic effects of central nervous system-directed gene therapy and bone marrow transplantation in the murine model of infantile neuronal ceroid lipofuscinosis. Ann. Neurol. 71, 797–804 (2012).
Roberts, M. S. et al. Combination small molecule PPT1 mimetic and CNS-directed gene therapy as a treatment for infantile neuronal ceroid lipofuscinosis. J. Inherit. Metab. Dis. 35, 847–857 (2012).
Macauley, S. L. et al. An anti-neuroinflammatory that targets dysregulated glia enhances the efficacy of CNS-directed gene therapy in murine infantile neuronal ceroid lipofuscinosis. J. Neurosci. 34, 13077–13082 (2014).
Shyng, C. et al. Synergistic effects of treating the spinal cord and brain in CLN1 disease. Proc. Natl Acad. Sci. USA 114, E5920–E5929 (2017).
Passini, M. A. et al. Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis. J. Neurosci. 26, 1334–1342 (2006).
Sondhi, D. et al. AAV2-mediated CLN2 gene transfer to rodent and non-human primate brain results in long-term TPP-I expression compatible with therapy for LINCL. Gene Ther. 12, 1618–1632 (2005).
Cabrera-Salazar, M. A. et al. Timing of therapeutic intervention determines functional and survival outcomes in a mouse model of late infantile Batten disease. Mol. Ther. 15, 1782–1788 (2007).
Sondhi, D. et al. Survival advantage of neonatal CNS gene transfer for late infantile neuronal ceroid lipofuscinosis. Exp. Neurol. 213, 18–27 (2008).
Katz, M. L. et al. AAV gene transfer delays disease onset in a TPP1-deficient canine model of the late infantile form of Batten disease. Sci. Transl. Med. 7, 313ra180 (2015).
Takahashi, K. et al. Gene therapy ameliorates spontaneous seizures associated with cortical neuron loss in a Cln2R207X mouse model. J. Clin. Invest. 133, e165908 (2023).
Tecedor, L. et al. An AAV variant selected through NHP screens robustly transduces the brain and drives secreted protein expression in NHPs and mice. Sci. Transl. Med. 17, eadr2531 (2025).
Mitchell, N. L. et al. Longitudinal in vivo monitoring of the CNS demonstrates the efficacy of gene therapy in a sheep model of CLN5 Batten disease. Mol. Ther. 26, 2366–2378 (2018).
Mitchell, N. L. et al. Long-term safety and dose escalation of intracerebroventricular CLN5 gene therapy in sheep supports clinical translation for CLN5 Batten disease. Front. Genet. 14, 1212228 (2023).
Murray, S. J. et al. Magnetic resonance imaging as a readout of CLN5 gene therapy efficacy in sheep. Brain Behav. 15, e70431 (2025).
Murray, S. J. et al. Intravitreal gene therapy protects against retinal dysfunction and degeneration in sheep with CLN5 Batten disease. Exp. Eye Res. 207, 108600 (2021).
Murray, S. J. et al. Efficacy of dual intracerebroventricular and intravitreal CLN5 gene therapy in sheep prompts the first clinical trial to treat CLN5 Batten disease. Front. Pharmacol. 14, 1212235 (2023).
Sondhi, D. et al. Partial correction of the CNS lysosomal storage defect in a mouse model of juvenile neuronal ceroid lipofuscinosis by neonatal CNS administration of an adeno-associated virus serotype rh.10 vector expressing the human CLN3 gene. Hum. Gene Ther. 25, 223–239 (2014).
Bosch, M. E. et al. Self-complementary AAV9 gene delivery partially corrects pathology associated with juvenile neuronal ceroid lipofuscinosis (CLN3). J. Neurosci. 36, 9669–9682 (2016).
Kleine et al. Gene therapy targeting the inner retina rescues the retinal phenotype in a mouse model of CLN3 Batten disease. Hum. Gene Ther. 31, 709–718 (2020).
Johnson, T. B. et al. Early postnatal administration of an AAV9 gene therapy is safe and efficacious in CLN3 disease. Front. Genet. 14, 1118649 (2023).
Ziółkowska, E. A. et al. Gene therapy ameliorates neuromuscular pathology in CLN3 disease. Acta Neuropathol. Commun. 13, 160 (2025).
Kleine et al. Prevention of photoreceptor cell loss in a Cln6nclf mouse model of Batten disease requires CLN6 gene transfer to bipolar cells. Mol. Ther. 26, 1343–1353 (2018).
Kleine et al. Neonatal brain-directed gene therapy rescues a mouse model of neurodegenerative CLN6 Batten disease. Hum. Mol. Genet. 28, 3867–3879 (2019).
Cain, J. T. et al. Gene therapy corrects brain and behavioral pathologies in CLN6-Batten disease. Mol. Ther. 27, 1836–1847 (2019).
White, K. A. et al. Intracranial delivery of AAV9 gene therapy partially prevents retinal degeneration and visual deficits in CLN6-Batten disease mice. Mol. Ther. Methods Clin. Dev. 20, 497–507 (2021).
Chen, X. et al. AAV9/MFSD8 gene therapy is effective in preclinical models of neuronal ceroid lipofuscinosis type 7 disease. J. Clin. Invest. 132, e146286 (2022).
Donsante, A. et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 317, 477 (2007).
Sabatino, D. E. et al. Evaluating the state of the science for adeno-associated virus integration: an integrated perspective. Mol. Ther. 30, 2646–2663 (2022).
Kim, J. et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl. J. Med. 381, 1644–1652 (2019).
Centa, J. L. et al. Therapeutic efficacy of antisense oligonucleotides in mouse models of CLN3 Batten disease. Nat. Med. 26, 1444–1451 (2020).
Zebronkysen. Fore Batten Foundation https://www.forebatten.org/zebronkysen (2024).
Pineda, M., Walterfang, M. & Patterson, M. C. Miglustat in Niemann-Pick disease type C patients: a review. Orphanet J. Rare Dis. 13, 140 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05174039 (2024).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Palmieri, M. et al. mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat. Commun. 8, 14338 (2017).
Lotfi, P. et al. Trehalose reduces retinal degeneration, neuroinflammation and storage burden caused by a lysosomal hydrolase deficiency. Autophagy 14, 1419–1434 (2018).
Soldati, C. et al. Repurposing of tamoxifen ameliorates CLN3 and CLN7 disease phenotype. EMBO Mol. Med. 13, e13742 (2021).
Seehafer, S. S. et al. Immunosuppression alters disease severity in juvenile Batten disease mice. J. Neuroimmunol. 230, 169–172 (2011).
Groh, J., Berve, K. & Martini, R. Fingolimod and teriflunomide attenuate neurodegeneration in mouse models of neuronal ceroid lipofuscinosis. Mol. Ther. 25, 1889–1899 (2017).
Tarczyluk-Wells, M. A. et al. Combined anti-inflammatory and neuroprotective treatments have the potential to impact disease phenotypes in Cln3 -/- mice. Front. Neurol. 10, 963 (2019).
Kovács, A. D. et al. Temporary inhibition of AMPA receptors induces a prolonged improvement of motor performance in a mouse model of juvenile Batten disease. Neuropharmacology 60, 405–409 (2011).
Kovács, A. D. et al. Age-dependent therapeutic effect of memantine in a mouse model of juvenile Batten disease. Neuropharmacology 63, 769–775 (2012).
Aldrich, A. et al. Efficacy of phosphodiesterase-4 inhibitors in juvenile Batten disease (CLN3). Ann. Neurol. 80, 909–923 (2016).
Ghosh, A., Rangasamy, S. B., Modi, K. K. & Pahan, K. Gemfibrozil, food and drug administration-approved lipid-lowering drug, increases longevity in mouse model of late infantile neuronal ceroid lipofuscinosis. J. Neurochem. 141, 423–435 (2017).
Sarkar, C. et al. Neuroprotection and lifespan extension in Ppt1(-/-) mice by NtBuHA: therapeutic implications for INCL. Nat. Neurosci. 16, 1608–1617 (2013).
Levin, S. W. et al. Oral cysteamine bitartrate and N-acetylcysteine for patients with infantile neuronal ceroid lipofuscinosis: a pilot study. Lancet Neurol. 13, 777–787 (2014).
Nickel, M. et al. Disease characteristics and progression in patients with late-infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease: an observational Cohort study. Lancet Child Adolesc. Health 2, 582–590 (2018).
Schulz, A. et al. Real-world clinical outcomes of patients with CLN2 disease treated with cerliponase alfa. Front. Neurol. 16, 1516026 (2025).
Schulz, A. et al. Safety and efficacy of cerliponase alfa in children with neuronal ceroid lipofuscinosis type 2 (CLN2 disease): an open-label extension study. Lancet Neurol. 23, 60–70 (2024).
Gaur, P. et al. Enzyme replacement therapy for CLN2 disease: MRI volumetry shows significantly slower volume loss compared with a natural history cohort. AJNR Am. J. Neuroradiol. 45, 1791–1797 (2024).
Schwering, C. et al. Development of the “Hamburg best practice guidelines for ICV-enzyme replacement therapy (ERT) in CLN2 disease” based on 6 years treatment experience in 48 patients. J. Child Neurol. 36, 635–641 (2021).
Schwering, C. et al. Therapeutic management of COVID-19 in a pediatric patient with neurodegenerative CLN2 disease and ICV-enzyme replacement therapy: a case report. Neuropediatrics 53, 381–384 (2022).
Dulz, S. et al. Ongoing retinal degeneration despite intraventricular enzyme replacement therapy with cerliponase alfa in late-infantile neuronal ceroid lipofuscinosis type 2 (CLN2 disease). Br. J. Ophthalmol. 107, 1478–1483 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05152914 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05791864 (2023).
Paushter, D. H. et al. The lysosomal function of progranulin, a guardian against neurodegeneration. Acta Neuropathol. 136, 1–17 (2018).
Jian, J., Hettinghouse, A. & Liu, C. J. Progranulin acts as a shared chaperone and regulates multiple lysosomal enzymes. Genes Dis. 4, 125–126 (2017).
Baker, M. et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919 (2006).
Laqtom, N. N. et al. CLN3 is required for the clearance of glycerophosphodiesters from lysosomes. Nature 609, 1005–1011 (2022).
Saarela, D. et al. Tagless LysoIP for immunoaffinity enrichment of native lysosomes from clinical samples. J. Clin. Invest. 135, e183592 (2024).
Nyame, K. et al. Glycerophosphodiesters inhibit lysosomal phospholipid catabolism in Batten disease. Mol. Cell. 84, 1354–1364.e9 (2024).
Medoh, U. N. et al. The Batten disease gene product CLN5 is the lysosomal bis(monoacylglycero)phosphate synthase. Science 381, 1182–1189 (2023).
Danyukova, T. et al. Loss of CLN7 results in depletion of soluble lysosomal proteins and impaired mTOR reactivation. Hum. Mol. Genet. 27, 1711–1722 (2018).
Huber, R. J., Mathavarajah, S. & Yap, S. Q. Mfsd8 localizes to endocytic compartments and influences the secretion of Cln5 and cathepsin D in dictyostelium. Cell Signal. 70, 109572 (2020).
Jansen, M. & Beaumelle, B. How palmitoylation affects trafficking and signaling of membrane receptors. Biol. Cell. 114, 61–72 (2022).
Jin, J., Zhi, X., Wang, X. & Meng, D. Protein palmitoylation and its pathophysiological relevance. J. Cell Physiol. 236, 3220–3233 (2021).
Ramzan, F., Abrar, F., Mishra, G. G., Qi Liao, L. M. & Martin, D. D. O. Lost in traffic: consequences of altered palmitoylation in neurodegeneration. Front. Physiol. 14, 1166125 (2023).
Petropavlovskiy, A. A., Kogut, J. A., Leekha, A., Townsend, C. A. & Sanders, S. S. A sticky situation: regulation and function of protein palmitoylation with a spotlight on the axon and axon initial segment. Neuronal Signal. 5, NS20210005 (2021).
Hayashi, T. Post-translational palmitoylation of ionotropic glutamate receptors in excitatory synaptic functions. Br. J. Pharmacol. 178, 784–797 (2021).
Tong, J. et al. GABAAR-PPT1 palmitoylation homeostasis controls synaptic transmission and circuitry oscillation. Transl. Psychiatry 14, 488 (2024).
Plavelil, N. et al. Defective anterograde protein-trafficking contributes to endoplasmic reticulum-stress in a CLN1 disease model. Neurobiol. Dis. 209, 106890 (2025).
Bagh, M. B. et al. Misrouting of v-ATPase subunit V0a1 dysregulates lysosomal acidification in a neurodegenerative lysosomal storage disease model. Nat. Commun. 8, 14612 (2017).
Bagh, M. B. et al. Disruption of lysosomal nutrient sensing scaffold contributes to pathogenesis of a fatal neurodegenerative lysosomal storage disease. J. Biol. Chem. 300, 105641 (2024).
Appu, A. P. et al. Niemann Pick C1 mistargeting disrupts lysosomal cholesterol homeostasis contributing to neurodegeneration in a Batten disease model. Sci. Adv. 11, eadr5703 (2025).
Barker, E. et al. Proximity labelling reveals effects of disease-causing mutation on the DNAJC5/cysteine string protein α interactome. Biochem. J. 481, 141–160 (2024).
Gorenberg, E. L. et al. Identification of substrates of palmitoyl protein thioesterase 1 highlights roles of depalmitoylation in disulfide bond formation and synaptic function. PLoS Biol. https://doi.org/10.1371/journal.pbio.3001590 (2022).
Dang, T. et al. ATP13A2 protects dopaminergic neurons in Parkinson’s disease: from biology to pathology. J. Biomed. Res. 36, 98–108 (2022).
van Veen, S. et al. ATP13A2 deficiency disrupts lysosomal polyamine export. Nature 578, 419–424 (2020).
Sharma, J. et al. Calpain activity is negatively regulated by a KCTD7-Cullin-3 complex via non-degradative ubiquitination. Cell Discov. 9, 32 (2023).
Wang, Y. et al. KCTD7 mutations impair the trafficking of lysosomal enzymes through CLN5 accumulation to cause neuronal ceroid lipofuscinoses. Sci. Adv. 8, eabm5578 (2022).
Palmer, D. N., Barry, L. A., Tyynelä, J. & Cooper, J. D. NCL disease mechanisms. Biochim. Biophys. Acta 1832, 1882–1893 (2013).
Cooper, J. D., Tarczyluk, M. A. & Nelvagal, H. R. Towards a new understanding of NCL pathogenesis. Biochim. Biophys. Acta 1852, 2256–2261 (2015).
Nelvagal, H. R., Lange, J., Takahashi, K., Tarczyluk-Wells, M. A. & Cooper, J. D. Pathomechanisms in the neuronal ceroid lipofuscinoses. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165570 (2020).
Takahashi, K., Nelvagal, H. R., Lange, J. & Cooper, J. D. Glial dysfunction and its contribution to the pathogenesis of the neuronal ceroid lipofuscinoses. Front. Neurol. 13, 886567 (2022).
Cooper, J. D., Messer, A., Feng, A. K., Chua-Couzens, J. & Mobley, W. C. Apparent loss and hypertrophy of interneurons in a mouse model of neuronal ceroid lipofuscinosis: evidence for partial response to insulin-like growth factor-1 treatment. J. Neurosci. 19, 2556–2567 (1999).
Chattopadhyay, S. et al. An autoantibody inhibitory to glutamic acid decarboxylase in the neurodegenerative disorder Batten disease. Hum. Mol. Genet. 11, 1421–1431 (2002).
Pontikis, C. C. et al. Late onset neurodegeneration in the Cln3-/- mouse model of juvenile neuronal ceroid lipofuscinosis is preceded by low level glial activation. Brain Res. 1023, 231–242 (2004).
Kopra, O. et al. A mouse model for Finnish variant late infantile neuronal ceroid lipofuscinosis, CLN5, reveals neuropathology associated with early aging. Hum. Mol. Genet. 13, 2893–2906 (2004).
Pontikis, C. C., Cotman, S. L., MacDonald, M. E. & Cooper, J. D. Thalamocortical neuron loss and localized astrocytosis in the Cln3Deltaex7/8 knock-in mouse model of Batten disease. Neurobiol. Dis. 20, 823–836 (2005).
Kielar, C. et al. Successive neuron loss in the thalamus and cortex in a mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 25, 150–162 (2007).
Oswald, M. J., Palmer, D. N., Kay, G. W., Barwell, K. J. & Cooper, J. D. Location and connectivity determine GABAergic interneuron survival in the brains of south Hampshire sheep with CLN6 neuronal ceroid lipofuscinosis. Neurobiol. Dis. 32, 50–65 (2008).
Partanen, S. et al. Synaptic changes in the thalamocortical system of cathepsin D-deficient mice: a model of human congenital neuronal ceroid-lipofuscinosis. J. Neuropathol. Exp. Neurol. 67, 16–29 (2008).
Morgan, J. P. et al. A murine model of variant late infantile ceroid lipofuscinosis recapitulates behavioral and pathological phenotypes of human disease. PLoS One 8, e78694 (2013).
Singh, Y. et al. Loss of Cln5 leads to altered Gad1 expression and deficits in interneuron development in mice. Hum. Mol. Genet. 28, 3309–3322 (2019).
Takahashi, K. et al. GABAergic interneurons contribute to the fatal seizure phenotype of CLN2 disease mice. JCI Insight https://doi.org/10.1172/jci.insight.184487 (2025).
Macauley, S. L. et al. Cerebellar pathology and motor deficits in the palmitoyl protein thioesterase 1-deficient mouse. Exp. Neurol. 217, 124–135 (2009).
Groh, J. et al. Immune cells perturb axons and impair neuronal survival in a mouse model of infantile neuronal ceroid lipofuscinosis. Brain 136, 1083–1101 (2013).
Bible, E., Gupta, P., Hofmann, S. L. & Cooper, J. D. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol. Dis. 16, 346–359 (2004).
Weimer, J. M. et al. Cerebellar defects in a mouse model of juvenile neuronal ceroid lipofuscinosis. Brain Res. 1266, 93–107 (2009).
Weimer, J. M. et al. Alterations in striatal dopamine catabolism precede loss of substantia nigra neurons in a mouse model of juvenile neuronal ceroid lipofuscinosis. Brain Res. 1162, 98–112 (2007).
Weimer, J. M. et al. Visual deficits in a mouse model of Batten disease are the result of optic nerve degeneration and loss of dorsal lateral geniculate thalamic neurons. Neurobiol. Dis. 22, 284–293 (2006).
Nelvagal, H. R., Dearborn, J. T., Ostergaard, J. R., Sands, M. S. & Cooper, J. D. Spinal manifestations of CLN1 disease start during the early postnatal period. Neuropathol. Appl. Neurobiol. 47, 251–267 (2021).
Ostergaard, J. R., Nelvagal, H. R. & Cooper, J. D. Top-down and bottom-up propagation of disease in the neuronal ceroid lipofuscinoses. Front. Neurol. 11, 1061363 (2022).
Gomez-Giro, G. et al. Synapse alterations precede neuronal damage and storage pathology in a human cerebral organoid model of CLN3-juvenile neuronal ceroid lipofuscinosis. Acta Neuropathol. Commun. 30, 222 (2019).
Ahrens-Nicklas, R. C. et al. Neuronal genetic rescue normalizes brain network dynamics in a lysosomal storage disorder despite persistent storage accumulation. Mol. Ther. 6, 2464–2473 (2022).
Lange, J. et al. Compromised astrocyte function and survival negatively impact neurons in infantile neuronal ceroid lipofuscinosis. Acta Neuropathol. Commun. 6, 74 (2018).
Parviainen, L. et al. Glial cells are functionally impaired in juvenile neuronal ceroid lipofuscinosis and detrimental to neurons. Acta Neuropathol. Commun. 5, 74 (2017).
Bosch, M. E. & Kielian, T. Astrocytes in juvenile neuronal ceroid lipofuscinosis (CLN3) display metabolic and calcium signaling abnormalities. J. Neurochem. 148, 612–624 (2019).
Burkovetskaya, M. et al. Evidence for aberrant astrocyte hemichannel activity in juvenile neuronal ceroid lipofuscinosis (JNCL). PLoS One 9, 95023 (2014).
Xiong, J. & Kielian, T. Microglia in juvenile neuronal ceroid lipofuscinosis are primed toward a pro-inflammatory phenotype. J. Neurochem. 127, 245–258 (2013).
Yasa, S. et al. Loss of CLN3 in microglia leads to impaired lipid metabolism and myelin turnover. Commun. Biol. 7, 1373 (2024).
Berve, K., West, B. L., Martini, R. & Groh, J. Sex- and region-biased depletion of microglia/macrophages attenuates CLN1 disease in mice. J. Neuroinflammation. 28, 323 (2020).
Macauley, S. L., Pekny, M. & Sands, M. S. The role of attenuated astrocyte activation in infantile neuronal ceroid lipofuscinosis. J. Neurosci. 26, 15575–15585 (2011).
Zhang, X. et al. Seizures in PPT1 Knock-in mice are associated with inflammatory activation of microglia. Int. J. Mol. Sci. 17, 5586 (2022).
Groh, J. et al. Sialoadhesin promotes neuroinflammation-related disease progression in two mouse models of CLN disease. Glia 64, 792–809 (2016).
Groh, J., Berve, K. & Martini, R. Immune modulation attenuates infantile neuronal ceroid lipofuscinosis in mice before and after disease onset. Brain Commun. 21, fcab047 (2021).
Haltia, M., Rapola, J. & Santavuori, P. Infantile type of so-called neuronal ceroid-lipofuscinosis. Histological and electron microscopic studies. Acta Neuropathol. 11, 157–170 (1973).
Rapola, J. & Haltia, M. Cytoplasmic inclusions in the vermiform appendix and skeletal muscle in two types of so-called neuronal ceroid-lipofuscinosis. Brain 96, 833–840 (1973).
Galvin, N. et al. A murine model of infantile neuronal ceroid lipofuscinosis-ultrastructural evaluation of storage in the central nervous system and viscera. Pediatr. Dev. Pathol. 11, 185–192 (2008).
Katz, M. L. et al. Extraneuronal pathology in a canine model of CLN2 neuronal ceroid lipofuscinosis after intracerebroventricular gene therapy that delays neurological disease progression. Gene Ther. 24, 215–223 (2017).
Ostergaard, J. R., Rasmussen, T. B. & Mølgaard, H. Cardiac involvement in juvenile neuronal ceroid lipofuscinosis (Batten disease). Neurology 76, 1245–1251 (2011).
Rietdorf, K. et al. Cardiac pathology in neuronal ceroid lipofuscinoses (NCL): more than a mere co-morbidity. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165643 (2020).
Ostergaard, J. R. Paroxysmal sympathetic hyperactivity in juvenile neuronal ceroid lipofuscinosis (Batten disease). Auton. Neurosci. 214, 15–18 (2018).
Handrup, M. M., Mølgaard, H., Andersen, B. N. & Ostergaard, J. R. Pacemaker implantation in juvenile neuronal ceroid lipofuscinosis (CLN3)-a long-term follow-up study. Front. Neurol. 13, 846240 (2022).
Baekmann, C. et al. Insight of autonomic dysfunction in CLN3 disease: a study on episodes resembling paroxysmal sympathetic hyperactivity (PSH). Orphanet J. Rare Dis. 19, 374 (2024).
Ostergaard, J. R. Etiology of anxious and fearful behavior in juvenile neuronal ceroid lipofuscinosis (CLN3 disease). Front. Psychiatry 14, 1059082 (2023).
Barney, C. C., Hoch, J., Byiers, B., Dimian, A. & Symons, F. J. A case-controlled investigation of pain experience and sensory function in neuronal ceroid lipofuscinosis. Clin. J. Pain. 31, 998–1003 (2015).
Mannerkoski, M. K., Heiskala, H. J., Santavuori, P. R. & Pouttu, J. A. Transdermal fentanyl therapy for pains in children with infantile neuronal ceroid lipofuscinosis. Eur. J. Paediatr. Neurol. 5, 175–177 (2001).
Santavuori, P. et al. Psychological symptoms and sleep disturbances in neuronal ceroid-lipofuscinoses (NCL). J. Inher. Metab. Dis. 16, 245–248 (1993).
Bosch-Queralt, M., Fledrich, R. & Stassart, R. M. Schwann cell functions in peripheral nerve development and repair. Neurobiol. Dis. 176, 105952 (2023).
Santosa, K. B., Keane, A. M., Jablonka-Shariff, A., Vannucci, B. & Snyder-Warwick, A. K. Clinical relevance of terminal Schwann cells: an overlooked component of the neuromuscular junction. J. Neurosci. Res. 96, 1125–1135 (2018).
Hastings, R. L. & Valdez, G. Origin, identity, and function of terminal Schwann cells. Trends Neurosci. 47, 432–446 (2024).
Ziółkowska, E. A. et al. Gene therapy prevents bowel dysmotility, enteric neuron degeneration and extends survival in lysosomal storage disorder mouse models. Sci. Transl. Med. 17, 1445 (2025).
Ziółkowska, E. A. et al. Enteric nervous system degeneration in human and murine CLN3 disease, is ameliorated by gene therapy in mice. Preprint at bioRxiv https://doi.org/10.1101/2025.01.29.635518 (2025).
Shacka, J. J. Mouse models of neuronal ceroid lipofuscinoses: useful pre-clinical tools to delineate disease pathophysiology and validate therapeutics. Brain Res. Bull. 1, 43–57 (2012).
Minnis, C. J., Thornton, C. D., FitzPatrick, L. M. & McKay, T. R. Cellular models of Batten disease. Biochim. Biophys. Acta Mol. Basis Dis. 1, 165559 (2020).
Nittari, G. et al. Batten disease through different in vivo and in vitro models: a review. J. Neurosci. Res. 101, 298–315 (2023).
Dwojak, E. et al. Six induced pluripotent stem cell lines from fibroblasts of individuals with CLN3-related conditions. Stem Cell Res. 81, 103563 (2024).
Otero, M. G. et al. Cellular modeling of CLN6 with IPSC-derived neurons and glia. Preprint at bioRxiv https://doi.org/10.1101/2024.01.29.577876 (2024).
Ofrim, M. et al. Characterization of two human induced pluripotent stem cell lines derived from Batten disease patient fibroblasts harbouring CLN5 mutations. Stem Cell Res. 74, 103291 (2024).
Bossolasco, P. et al. GRN-/- iPSC-derived cortical neurons recapitulate the pathological findings of both frontotemporal lobar degeneration and neuronal ceroidolipofuscinosis. Neurobiol. Dis. 175, 105891 (2022).
Uusi-Rauva, K. et al. Induced pluripotent stem cells derived from a CLN5 patient manifest phenotypic characteristics of neuronal ceroid lipofuscinoses. Int. J. Mol. Sci. 1, 955 (2017).
Lojewski, X. et al. Human iPSC models of neuronal ceroid lipofuscinosis capture distinct effects of TPP1 and CLN3 mutations on the endocytic pathway. Hum. Mol. Genet. 23, 2005–2022 (2014).
Kinarivala, N. et al. An iPSC-derived neuron model of CLN3 disease facilitates small molecule phenotypic screening. ACS Pharmacol. Transl. Sci. 3, 931–947 (2020).
Mikulka, C. R. et al. Cell-autonomous expression of the acid hydrolase galactocerebrosidase. Proc. Natl Acad. Sci. USA 21, 9032–9041 (2020).
Eaton, S. L. & Wishart, T. M. Bridging the gap: large animal models in neurodegenerative research. Mamm. Genome 28, 324–337 (2017).
Katz, M. L. et al. Canine neuronal ceroid lipofuscinoses: promising models for preclinical testing of therapeutic interventions. Neurobiol. Dis. 108, 277–287 (2017).
Murray, S. J. & Mitchell, N. L. The translational benefits of sheep as large animal models of human neurological disorders. Front. Vet. Sci. 15, 831838 (2022).
Mitchell, N. L., Russell, K. N., Barrell, G. K., Tammen, I. & Palmer, D. N. Characterization of neuropathology in ovine CLN5 and CLN6 neuronal ceroid lipofuscinoses (Batten disease). Dev. Neurobiol. 83, 127–142 (2023).
Murray, S. J. et al. Progressive MRI brain volume changes in ovine models of CLN5 and CLN6 neuronal ceroid lipofuscinosis. Brain Commun. 2, fcac339 (2023).
Kay, G. W., Palmer, D. N., Rezaie, P. & Cooper, J. D. Activation of non-neuronal cells within the prenatal developing brain of sheep with neuronal ceroid lipofuscinosis. Brain Pathol. 16, 110–116 (2006).
Oswald, M. J. et al. Glial activation spreads from specific cerebral foci and precedes neurodegeneration in presymptomatic ovine neuronal ceroid lipofuscinosis (CLN6). Neurobiol. Dis. 20, 49–63 (2005).
Amorim, I. S. et al. Molecular neuropathology of the synapse in sheep with CLN5 Batten disease. Brain Behav. 9, e00401 (2015).
Frugier, T. et al. A new large animal model of CLN5 neuronal ceroid lipofuscinosis in Borderdale sheep is caused by a nucleotide substitution at a consensus splice site (c.571+1G>A) leading to excision of exon 3. Neurobiol. Dis. 29, 306–315 (2008).
Munesue, Y. et al. Cynomolgus macaque model of neuronal ceroid lipofuscinosis type 2 disease. Exp. Neurol. 363, 114381 (2023).
McBride, J. L. et al. Discovery of a CLN7 model of Batten disease in non-human primates. Neurobiol. Dis. 119, 65–78 (2018).
Swier, V. J. et al. A novel porcine model of CLN2 Batten disease that recapitulates patient phenotypes. Neurotherapeutics 19, 1905–1919 (2022).
Knoernschild, K. et al. Magnetic resonance brain volumetry biomarkers of CLN2 Batten disease identified with miniswine model. Sci. Rep. 29, 5146 (2023).
Swier, V. J. et al. A novel porcine model of CLN3 Batten disease recapitulates clinical phenotypes. Dis. Model. Mech. 1, dmm050038 (2023).
Eaton, S. L. et al. CRISPR/Cas9 mediated generation of an ovine model for infantile neuronal ceroid lipofuscinosis (CLN1 disease). Sci. Rep. 9, 9891 (2019).
Eaton, S. L. et al. Modelling neurological diseases in large animals: criteria for model selection and clinical assessment. Cells 11, 2641 (2022).
Iwan, K. et al. Urine proteomics analysis of patients with neuronal ceroid lipofuscinoses. iScience 31, 102020 (2020).
Brudvig, J. J. et al. Glycerophosphoinositol is elevated in blood samples from CLN3Δex7-8 pigs, Cln3Δex7-8 mice, and CLN3-affected individuals. Biomark. Insights 17, 11772719221107765 (2022).
Velinov, M. et al. Mutations in the gene DNAJC5 cause autosomal dominant Kufs disease in a proportion of cases: study of the Parry family and 8 other families. PLoS One 7, 29729 (2012).
Sidransky, E. et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 22, 1651–1661 (2009).
Gan-Or, Z. et al. The p.L302P mutation in the lysosomal enzyme gene SMPD1 is a risk factor for Parkinson disease. Neurology 23, 1606–1610 (2013).
Michelakakis, H. et al. Evidence of an association between the scavenger receptor class B member 2 gene and Parkinson’s disease. Mov. Disord. 27, 400–405 (2012).
Li, G. et al. Association of GALC, ZNF184, IL1R2 and ELOVL7 with Parkinson’s disease in southern Chinese. Front. Aging Neurosci. 13, 402 (2018).
Lopergolo, D. et al. Familial Alzheimer’s disease associated with heterozygous NPC1 mutation. J. Med. Genet. 21, 332–339 (2024).
Suire, C. N. et al. Cathepsin D regulates cerebral Aβ42/40 ratios via differential degradation of Aβ42 and Aβ40. Alzheimers Res. Ther. 6, 80 (2020).
Ntais, C., Polycarpou, A. & Ioannidis, J. P. Meta-analysis of the association of the cathepsin D Ala224Val gene polymorphism with the risk of Alzheimer’s disease: a HuGE gene-disease association review. Am. J. Epidemiol. 15, 527–536 (2004).
Solé-Domènech, S. et al. Lysosomal enzyme tripeptidyl peptidase 1 destabilizes fibrillar Aβ by multiple endoproteolytic cleavages within the β-sheet domain. Proc. Natl Acad. Sci. Usa. 13, 1493–1498 (2018).
Benitez, B. A. et al. Haploinsufficiency of lysosomal enzyme genes in Alzheimer’s disease. Preprint at bioRxiv https://doi.org/10.1101/2024.11.16.623962 (2024).
Gupta, P. et al. Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc. Natl Acad. Sci. USA 98, 13566–13571 (2001).
Miller, J. N., Kovács, A. D. & Pearce, D. A. The novel Cln1(R151X) mouse model of infantile neuronal ceroid lipofuscinosis (INCL) for testing nonsense suppression therapy. Hum. Mol. Genet. 24, 185–196 (2015).
Jalanko, A. et al. Mice with Ppt1Deltaex4 mutation replicate the INCL phenotype and show an inflammation-associated loss of interneurons. Neurobiol. Dis. 18, 226–241 (2005).
Sanders, D. N. et al. A mutation in canine PPT1 causes early onset neuronal ceroid lipofuscinosis in a Dachshund. Mol. Genet. Metab. 100, 349–356 (2010).
Kolicheski, A. et al. Homozygous PPT1 splice donor mutation in a cane corso dog with neuronal ceroid lipofuscinosis. J. Vet. Intern. Med. 31, 149–157 (2017).
Sleat, D. E. et al. A mouse model of classical late-infantile neuronal ceroid lipofuscinosis based on targeted disruption of the CLN2 gene results in a loss of tripeptidyl-peptidase I activity and progressive neurodegeneration. J. Neurosci. 24, 9117–9126 (2004).
Geraets, R. D. et al. A tailored mouse model of CLN2 disease: a nonsense mutant for testing personalized therapies. PLoS One 12, e0176526 (2017).
Awano, T. et al. A frame shift mutation in canine TPP1 (the ortholog of human CLN2) in a juvenile Dachshund with neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 89, 254–260 (2006).
Mahmood, F. et al. A zebrafish model of CLN2 disease is deficient in tripeptidyl peptidase 1 and displays progressive neurodegeneration accompanied by a reduction in proliferation. Brain 136, 1488–1507 (2013).
Mitchison, H. M. et al. Targeted disruption of the Cln3 gene provides a mouse model for Batten disease. The Batten mouse model consortium [corrected]. Neurobiol. Dis. 6, 321–334 (1999).
Katz, M. L. et al. A mouse gene knockout model for juvenile ceroid-lipofuscinosis (Batten disease). J. Neurosci. Res. 57, 551–556 (1999).
Cotman, S. L. et al. Cln3(Deltaex7/8) knock-in mice with the common JNCL mutation exhibit progressive neurologic disease that begins before birth. Hum. Mol. Genet. 11, 2709–2721 (2002).
Heins-Marroquin, U. et al. CLN3 deficiency leads to neurological and metabolic perturbations during early development. Life Sci. Alliance 7, e202302057 (2024).
Wager, K. et al. Neurodegeneration and epilepsy in a zebrafish model of CLN3 disease (Batten disease). PLoS One 11, e0157365 (2016).
López-Begines, S. et al. Neuronal lipofuscinosis caused by Kufs disease/CLN4 DNAJC5 mutations but not by a CSPα/DNAJC5 deficiency. Sci. Adv. 11, eads3393 (2025).
Melville, S. A. et al. A mutation in canine CLN5 causes neuronal ceroid lipofuscinosis in border collie dogs. Genomics 86, 287–294 (2005).
Kolicheski, A. et al. Australian cattle dogs with neuronal ceroid lipofuscinosis are homozygous for a CLN5 nonsense mutation previously identified in border collies. J. Vet. Intern. Med. 30, 1149–1158 (2016).
Gilliam, D. et al. Golden retriever dogs with neuronal ceroid lipofuscinosis have a two-base-pair deletion and frameshift in CLN5. Mol. Genet. Metab. 115, 101–109 (2015).
Houweling, P. J. et al. Neuronal ceroid lipofuscinosis in Devon cattle is caused by a single base duplication (c.662dupG) in the bovine CLN5 gene. Biochim. Biophys. Acta 1762, 890–897 (2006).
Bronson, R. T. et al. Neuronal ceroid lipofuscinosis (nclf), a new disorder of the mouse linked to chromosome 9. Am. J. Med. Genet. 77, 289–297 (1998).
Jolly, R. D. et al. Ceroid-lipofuscinosis (Batten’s disease): pathogenesis and sequential neuropathological changes in the ovine model. Neuropathol. Appl. Neurobiol. 15, 371–383 (1989).
Katz, M. L. et al. O’Brien DP. A missense mutation in canine CLN6 in an Australian shepherd with neuronal ceroid lipofuscinosis. J. Biomed. Biotechnol. 2011, 198042 (2011).
Brandenstein, L., Schweizer, M., Sedlacik, J., Fiehler, J. & Storch, S. Lysosomal dysfunction and impaired autophagy in a novel mouse model deficient for the lysosomal membrane protein Cln7. Hum. Mol. Genet. 25, 777–791 (2016).
Ashwini, A. et al. Neuronal ceroid lipofuscinosis associated with an MFSD8 mutation in Chihuahuas. Mol. Genet. Metab. 118, 326–332 (2016).
Faller, K. M. et al. The Chihuahua dog: a new animal model for neuronal ceroid lipofuscinosis CLN7 disease? J. Neurosci. Res. 94, 339–347 (2016).
Katz, M. L. et al. A mutation in the CLN8 gene in English Setter dogs with neuronal ceroid-lipofuscinosis. Biochem. Biophys. Res. Commun. 327, 541–547 (2005).
Guo, J. et al. A CLN8 nonsense mutation in the whole genome sequence of a mixed breed dog with neuronal ceroid lipofuscinosis and Australian shepherd ancestry. Mol. Genet. Metab. 112, 302–309 (2014).
Guo, J. et al. Neuronal ceroid lipofuscinosis in a German shorthaired pointer associated with a previously reported CLN8 nonsense variant. Mol. Genet. Metab. Rep. 21, 100521 (2019).
Saftig, P. et al. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 14, 3599–3608 (1995).
Awano, T. et al. A mutation in the cathepsin D gene (CTSD) in American bulldogs with neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 87, 341–348 (2006).
Tyynelä, J. et al. A mutation in the ovine cathepsin D gene causes a congenital lysosomal storage disease with profound neurodegeneration. EMBO J. 19, 2786–2792 (2000).
Hafler, B. P., Klein, Z. A., Jimmy Zhou, Z. & Strittmatter, S. M. Progressive retinal degeneration and accumulation of autofluorescent lipopigments in progranulin deficient mice. Brain Res. 1588, 168–174 (2014).
Schultheis, P. J. et al. Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited α-synuclein accumulation and age-dependent sensorimotor deficits. Hum. Mol. Genet. 22, 2067–2082 (2013).
Farias, F. H. et al. A truncating mutation in ATP13A2 is responsible for adult-onset neuronal ceroid lipofuscinosis in Tibetan terriers. Neurobiol. Dis. 42, 468–474 (2011).
Liang, J. H. et al. Kctd7 deficiency induces myoclonic seizures associated with purkinje cell death and microvascular defects. Dis. Model. Mech. 15, dmm049642 (2022).
Acknowledgements
Our work is inspired by families affected by Batten disease, and this manuscript is dedicated to them and their children. We would also like to thank the many talented individuals from our labs who have contributed so much to our research efforts. We also thank A. Barnwell for constructive comments on the manuscript. Studies in our laboratories were funded by many sources, including NIH grants R56 NS117635, R01 NS124655, R01 NS140682, R21 NS116574, R21 NS126907 and RM1 NS132962 (to J.D.C.). Foundation support was obtained from the Batten Disease Support Research and Advocacy Foundation (BDSRA Foundation), Batten Disease Family Association (BDFA), Batten Disease Global Research Initiative, Beyond Batten Disease Foundation, Children’s Brain Diseases Foundation, Fore Batten Foundation, Haley’s Heroes, Lehrman Family Fund, Noah’s Hope/Hope for Bridget and The Natalie Fund.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article. All authors contributed substantially to discussion of the content. J.D.C. wrote the first draft of the article but all authors reviewed and/or substantially edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.D.C. has received research support from Abeona Therapeutics Inc., BioMarin Pharmaceutical Inc., Neurogene, and REGENXBIO Inc. and is a consultant for JCR Pharmaceuticals. The remaining authors declare no conflicts of interest.
Peer review
Peer review information
Nature Reviews Neurology thanks Paul Gissen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
The Batten Disease Support Research and Advocacy Foundation (BDSRA Foundation): https://bdsrafoundation.org
The NCL Resource - A gateway for Batten disease: https://www.ucl.ac.uk/ncl-disease/ncl-resource-gateway-batten-disease
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ziółkowska, E.A., Takahashi, K., Dickson, P.I. et al. Neuronal ceroid lipofuscinosis: underlying mechanisms and emerging therapeutic targets. Nat Rev Neurol 21, 606–622 (2025). https://doi.org/10.1038/s41582-025-01132-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41582-025-01132-4
This article is cited by
-
Enteric nervous system degeneration in human and murine CLN3 disease, is ameliorated by gene therapy in mice
Acta Neuropathologica Communications (2025)
-
Limited therapeutic efficacy of N-acetyl-L-leucine in a mouse model of CLN1 disease
Scientific Reports (2025)