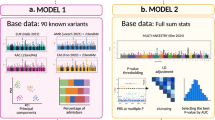
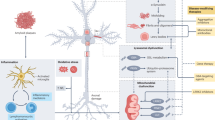
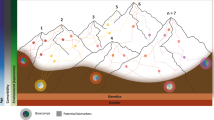
Abstract
The global burden of Parkinson disease (PD) is rapidly shifting towards low-income and middle-income countries (LMICs), which already account for 44% of all individuals with PD. Despite this trend, the populations of LMICs and other under-represented populations defined by ethnicity, sex, geography and minority groups within high-income countries remain largely excluded from PD research. The continuation of these disparities limits our knowledge of disease biology and restricts the applicability of advances in prevention, diagnosis and treatment, increasing inequity in global health. Substantial disparities persist across the PD research continuum, extending beyond resource limitations and encompassing epidemiology, environment, genetics, deep phenotyping and biomarkers, data integration and diversity-aware analytics, clinical trials and basic science. To repair structural diversity gaps that compromise validity and equity in PD research, we propose an ethically grounded, coordinated agenda that prioritizes increased funding and local capacity building in under-represented regions, the development and adoption of harmonized and context-adapted methods, sustained community engagement with cultural competence, the creation of collaborative research networks, and more inclusive editorial and regulatory policies. Moreover, because inequities in care and research commonly co-occur and are mutually reinforcing, PD research should advance alongside a worldwide commitment to minimum standards of care and access to treatment. This Perspective details diversity gaps across PD research, outlines priority actions to address them, and illustrates, through recent examples, how interventions along these axes can advance equity and representation in PD studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Raudino, F. The Parkinson disease before James Parkinson. Neurol. Sci. 33, 945–948 (2012).
GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953 (2018).
Su, D. et al. Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: modelling study of Global Burden of Disease Study 2021. Br. Med. J. 388, e080952 (2025).
Schlickmann, T. H. et al. Prevalence, distribution and future projections of Parkinson disease in Brazil: insights from the ELSI-Brazil cohort study. Lancet Reg. Health Am. 44, 101046 (2025).
Schiess, N. et al. Six action steps to address global disparities in Parkinson disease: a World Health Organization priority. JAMA Neurol. 79, 929–936 (2022).
Ben-Shlomo, Y. et al. The epidemiology of Parkinson’s disease. Lancet 403, 283–292 (2024).
Pereira, G. M. et al. A systematic review and meta-analysis of the prevalence of Parkinson’s disease in lower to upper-middle-income countries. npj Parkinsons Dis. 10, 181 (2024).
Ou, Z. et al. Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories from 1990 to 2019. Front. Public Health 9, 776847 (2021).
Zhu, J. et al. Temporal trends in the prevalence of Parkinson’s disease from 1980 to 2023: a systematic review and meta-analysis. Lancet Healthy Longev. 5, e464–e479 (2024).
Siddiqi, S. et al. Race and ethnicity matter! Moving Parkinson’s risk research towards diversity and inclusiveness. npj Parkinsons Dis. 11, 45 (2025).
Pearson, C. et al. Care access and utilization among medicare beneficiaries living with Parkinson’s disease. npj Parkinsons Dis. 9, 108 (2023).
Okubadejo, N. U., Bower, J. H., Rocca, W. A. & Maraganore, D. M. Parkinson’s disease in Africa: a systematic review of epidemiologic and genetic studies. Mov. Disord. 21, 2150–2156 (2006).
Yang, Q. et al. Disease burden of Parkinson’s disease in Asia and its 34 countries and territories from 1990 to 2021: findings from the Global Burden of Disease Study 2021. Neuroepidemiology 59, 525–557 (2024).
Wright Willis, A., Evanoff, B. A., Lian, M., Criswell, S. R. & Racette, B. A. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology 34, 143–151 (2010).
Dahodwala, N. et al. Racial differences in the diagnosis of Parkinson’s disease. Mov. Disord. 24, 1200–1205 (2009).
Willis, A. W. et al. Incidence of Parkinson disease in North America. npj Parkinsons Dis. 8, 170 (2022).
Harris, S., Narayanan, N. S. & Tranel, D. Does Black vs. White race affect practitioners’ appraisal of Parkinson’s disease? npj Parkinsons Dis. 9, 106 (2023).
Dekker, M. C. J. et al. Parkinson’s disease research on the African continent: obstacles and opportunities. Front. Neurol. 11, 512 (2020).
Ma, E., Krening, E., Seto, B. K. & Bruno, M. K. Challenges faced by rural health care providers caring for Parkinson’s disease patients in neighbor islands of Hawai’i. Hawaii J. Health Soc. Welf. 83, 99–107 (2024).
Wan, Y. et al. Determinants of diagnostic latency in Chinese people with Parkinson’s disease. BMC Neurol. 19, 120 (2019).
Tan, A. H. et al. Knowledge of Parkinson’s disease in a multiethnic urban Asian setting. J. Parkinsons Dis. 5, 865–879 (2015).
George, S., Duran, N. & Norris, K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public Health 104, e16–e31 (2014).
Armstrong, M. J. & Okun, M. S. Time for a new image of Parkinson disease. JAMA Neurol. 77, 1345–1346 (2020).
Zirra, A. et al. Gender differences in the prevalence of Parkinson’s disease. Mov. Disord. Clin. Pract. 10, 86–93 (2023).
Park, J. H. et al. Trends in the incidence and prevalence of Parkinson’s disease in Korea: a nationwide, population-based study. BMC Geriatr. 19, 320 (2019).
Kimura, H. et al. Female preponderance of Parkinson’s disease in Japan. Neuroepidemiology 21, 292–296 (2002).
Kapoor, M. et al. Missing female patients: an observational analysis of sex ratio among outpatients in a referral tertiary care public hospital in India. BMJ Open 9, e026850 (2019).
Tan, A. H. et al. Genetic testing for Parkinson’s disease and movement disorders in less privileged areas: barriers and opportunities. Mov. Disord. Clin. Pract. 11, 14–20 (2024).
Abraham, D. S. et al. Sex differences in Parkinson’s disease presentation and progression. Parkinsonism Relat. Disord. 69, 48–54 (2019).
Subramanian, I. et al. Unmet needs of women living with Parkinson’s disease: gaps and controversies. Mov. Disord. 37, 444–455 (2022).
Sarapura-Castro, E. et al. Prevalence of Parkinson’s disease in a town of the Central Highlands of Peru: a population-based study. Mov. Disord. Clin. Pract. https://doi.org/10.1002/mdc3.70354 (2025).
Bonander, C. et al. A capture–recapture-based ascertainment probability weighting method for effect estimation with under-ascertained outcomes. Epidemiology 35, 340–348 (2024).
García-Bustillo, Á. et al. The feasibility and practical utility of virtual visits for patients with Parkinson’s disease in different world regions. Mov. Disord. Clin. Pract. https://doi.org/10.1002/mdc3.70314 (2025).
Charani, E. et al. Funders: the missing link in equitable global health research? PLoS Glob. Public Health 2, e0000583 (2022).
Dorsey, E. R. & Bloem, B. R. Parkinson’s disease is predominantly an environmental disease. J. Parkinsons Dis. 14, 451–465 (2024).
Schreinemachers, P. & Tipraqsa, P. Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 37, 616–626 (2012).
Siddique, H. M. A. & Kiani, A. K. Industrial pollution and human health: evidence from middle-income countries. Environ. Sci. Pollut. Res. Int. 27, 12439–12448 (2020).
Tangamornsuksan, W. et al. Paraquat exposure and Parkinson’s disease: a systematic review and meta-analysis. Arch. Environ. Occup. Health 74, 225–238 (2019).
Kasdagli, M. I., Katsouyanni, K., Dimakopoulou, K. & Samoli, E. Air pollution and Parkinson’s disease: a systematic review and meta-analysis up to 2018. Int. J. Hyg. Environ. Health 222, 402–409 (2019).
Lock, E. A., Zhang, J. & Checkoway, H. Solvents and Parkinson disease: a systematic review of toxicological and epidemiological evidence. Toxicol. Appl. Pharmacol. 266, 345–355 (2013).
Zhao, Y., Ray, A., Portengen, L., Vermeulen, R. & Peters, S. Metal exposure and risk of Parkinson disease: a systematic review and meta-analysis. Am. J. Epidemiol. 192, 1207–1223 (2023).
Fang, X. et al. Association of levels of physical activity with risk of Parkinson disease: a systematic review and meta-analysis. JAMA Netw. Open 1, e182421 (2018).
Costa, J., Lunet, N., Santos, C., Santos, J. & Vaz-Carneiro, A. Caffeine exposure and the risk of Parkinson’s disease: a systematic review and meta-analysis of observational studies. J. Alzheimers Dis. 20 (Suppl. 1), S221–S238 (2010).
Strikwerda, A. J., Dommershuijsen, L. J., Ikram, M. K. & Voortman, T. Diet quality and risk of Parkinson’s disease: the Rotterdam Study. Nutrients 13, 3970 (2021).
Jafari, S., Etminan, M., Aminzadeh, F. & Samii, A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov. Disord. 28, 1222–1229 (2013).
Moura, D. D. et al. History of high household pesticide use and Parkinson’s disease in Brazil. Parkinsonism Relat. Disord. 113, 105493 (2023).
Santos-Lobato, B. L. & Schuh, A. F. S. Exposure to household pesticides and Parkinson’s disease in the Parkinson’s Progression Markers Initiative cohort. Front. Neurol. 15, 1411468 (2024).
Ritz, B. et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch. Neurol. 64, 990–997 (2007).
Lee, P. C., Rhodes, S. L., Sinsheimer, J. S., Bronstein, J. & Ritz, B. Functional paraoxonase 1 variants modify the risk of Parkinson’s disease due to organophosphate exposure. Environ. Int. 56, 42–47 (2013).
Kumar, B. et al. Role of PON1 L55M gene polymorphism in Parkinson’s disease among North Indian population. Neurol. India 72, 364–367 (2024).
Najafi, F. et al. Association between socioeconomic status and Parkinson’s disease: findings from a large incident case-control study. BMJ Neurol. Open 5, e000386 (2023).
Atterling Brolin, K. et al. Environmental risk factors for Parkinson’s disease: a critical review and policy implications. Mov. Disord. 40, 204–221 (2025).
Mills, M. C. & Rahal, C. The GWAS Diversity Monitor tracks diversity by disease in real time. Nat. Genet. 52, 242–243 (2020).
Jia, F., Fellner, A. & Kumar, K. R. Monogenic Parkinson’s disease: genotype, phenotype, pathophysiology, and genetic testing. Genes 13, 471 (2022).
Vollstedt, E. J. et al. Embracing monogenic Parkinson’s disease: the MJFF Global Genetic PD Cohort. Mov. Disord. 38, 286–303 (2023).
Saffie Awad, P. et al. Frequency of hereditary and GBA1-related Parkinsonism in Latin America: a systematic review and meta-analysis. Mov. Disord. 39, 6–16 (2024).
Shu, L., Zhang, Y., Sun, Q., Pan, H. & Tang, B. A comprehensive analysis of population differences in variant distribution in Parkinson’s disease. Front. Aging Neurosci. 11, 13 (2019).
Schumacher-Schuh, A. F. et al. Underrepresented populations in Parkinson’s genetics research: current landscape and future directions. Mov. Disord. 37, 1593–1604 (2022).
Koros, C. et al. The landscape of monogenic Parkinson’s disease in populations of non-European ancestry: a narrative review. Genes 14, 2097 (2023).
Junker, J. et al. Team science approaches to unravel monogenic Parkinson’s disease on a global scale. Mov. Disord. 39, 1868–1873 (2024).
Guadagnolo, D., Piane, M., Torrisi, M. R., Pizzuti, A. & Petrucci, S. Genotype–phenotype correlations in monogenic Parkinson disease: a review on clinical and molecular findings. Front. Neurol. 12, 648588 (2021).
Foo, J. N. et al. Identification of risk loci for Parkinson disease in Asians and comparison of risk between Asians and Europeans: a genome-wide association study. JAMA Neurol. 77, 746–754 (2020).
Akçimen, F. et al. Large-scale genetic characterization of Parkinson’s disease in the African and African admixed populations. Preprint at medRxiv https://doi.org/10.1101/2025.01.14.25320205 (2025).
Lim, S. Y. et al. Uncovering the genetic basis of Parkinson’s disease globally: from discoveries to the clinic. Lancet Neurol. 23, 1267–1280 (2024).
Khani, M. et al. Towards a global view of Parkinson’s disease genetics. Ann. Neurol. 95, 831–842 (2024).
Tan, A. H. et al. Global perspectives on returning genetic research results in Parkinson disease. Neurol. Genet. 10, e200213 (2024).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
Tan, M. M. X. et al. Genome-wide determinants of mortality and motor progression in Parkinson’s disease. npj Parkinsons Dis. 10, 113 (2024).
Martínez Carrasco, A. et al. Genome-wide analysis of motor progression in Parkinson disease. Neurol. Genet. 9, e200092 (2023).
Global Parkinson’s Genetics Program. GP2: the Global Parkinson’s Genetics Program. Mov. Disord. 36, 842–851 (2021).
Zabetian, C. P. & Mata, I. F. Latin American Research Consortium on the Genetics of PD (LARGE-PD). LARGE-PD: examining the genetics of Parkinson’s disease in Latin America. Mov. Disord. 32, 1330–1331 (2017).
International Parkinson Disease Genomics Consortium (IPDGC). Ten years of the International Parkinson Disease Genomics Consortium: progress and next steps. J. Parkinsons Dis. 10, 19–30 (2020).
Mok, K. Y. East Asian Parkinson Disease Genomics Consortium. The East Asian Parkinson Disease Genomics Consortium. Lancet Neurol. 20, 982 (2021).
Loesch, D. P. et al. Characterizing the genetic architecture of Parkinson’s disease in Latinos. Ann. Neurol. 90, 353–365 (2021).
Rajan, R. et al. Genetic architecture of Parkinson’s disease in the Indian population: harnessing genetic diversity to address critical gaps in Parkinson’s disease research. Front. Neurol. 11, 524 (2020).
Step, K. et al. Genome-wide association analyses reveal susceptibility variants linked to Parkinson’s disease in the South African population using inferred global and local ancestry. Preprint at medRxiv https://doi.org/10.1101/2025.08.01.25331910 (2025).
Kim, J. J. et al. Multi-ancestry genome-wide association meta-analysis of Parkinson’s disease. Nat. Genet. 56, 27–36 (2024).
Rizig, M. et al. Identification of genetic risk loci and causal insights associated with Parkinson’s disease in African and African admixed populations: a genome-wide association study. Lancet Neurol. 22, 1015–1025 (2023).
Simón-Sánchez, J. et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 41, 1308–1312 (2009).
Saffie-Awad, P. et al. Insights into ancestral diversity in Parkinson’s disease risk: a comparative assessment of polygenic risk scores. npj Parkinsons Dis. 11, 201 (2025).
Kachuri, L. et al. Principles and methods for transferring polygenic risk scores across global populations. Nat. Rev. Genet. 25, 8–25 (2024).
Klokkaris, A. & Migdalska-Richards, A. An overview of epigenetic changes in the Parkinson’s disease brain. Int. J. Mol. Sci. 25, 6168 (2024).
Lefèvre-Arbogast, S. et al. Assessing the contribution of the chemical exposome to neurodegenerative disease. Nat. Neurosci. 27, 812–821 (2024).
Fatumo, S. et al. A roadmap to increase diversity in genomic studies. Nat. Med. 28, 243–250 (2022).
Rebbeck, T. R. et al. A framework for promoting diversity, equity, and inclusion in genetics and genomics research. JAMA Health Forum 3, e220603 (2022).
Bhérer, C. et al. A cost-effective sequencing method for genetic studies combining high-depth whole exome and low-depth whole genome. npj Genom. Med. 9, 8 (2024).
Sengupta, D. et al. Performance and accuracy evaluation of reference panels for genotype imputation in sub-Saharan African populations. Cell Genom. 3, 100332 (2023).
Nicol, D., Nielsen, J. & Archer, M. Data access arrangements in genomic research consortia. Sci. Rep. 14, 21685 (2024).
Blauwendraat, C. et al. Tackling a disease on a global scale, the Global Parkinson’s Genetics Program, GP2: a new generation of opportunities. Am. J. Hum. Genet. 112, 1988–2000 (2025).
Heinzel, S., Lerche, S., Maetzler, W. & Berg, D. Global, yet incomplete overview of cohort studies in Parkinson’s disease. J. Parkinsons Dis. 7, 423–432 (2017).
Marek, K. et al. The Parkinson’s progression markers initiative (PPMI) — establishing a PD biomarker cohort. Ann. Clin. Transl. Neurol. 5, 1460–1477 (2018).
Rosenthal, L. S. et al. The NINDS Parkinson’s disease biomarkers program. Mov. Disord. 31, 915–923 (2016).
Pourzinal, D. et al. Systematic review of data-driven cognitive subtypes in Parkinson disease. Eur. J. Neurol. 29, 3395–3417 (2022).
Mestre, T. A. et al. Parkinson’s disease subtypes: critical appraisal and recommendations. J. Parkinsons Dis. 11, 395–404 (2021).
Bega, D. et al. Clinical utility of DaTscan in patients with suspected Parkinsonian syndrome: a systematic review and meta-analysis. npj Parkinsons Dis. 7, 43 (2021).
Siderowf, A. et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 22, 407–417 (2023).
Pedersen, C. C. et al. Serum neurofilament light at diagnosis: a prognostic indicator for accelerated disease progression in Parkinson’s disease. npj Parkinsons Dis. 10, 162 (2024).
Mao, H. et al. Ultrasensitive detection of aggregated α-synuclein using quiescent seed amplification assay for the diagnosis of Parkinson’s disease. Transl. Neurodegener. 13, 35 (2024).
Kuang, Y. et al. A skin-specific α-synuclein seeding amplification assay for diagnosing Parkinson’s disease. npj Parkinsons Dis. 10, 129 (2024).
Alonso, C. C. G., Silva, F. G., Costa, L. O. P. & Freitas, S. M. S. F. Smell tests to distinguish Parkinson’s disease from other neurological disorders: a systematic review and meta-analysis. Expert Rev. Neurother. 21, 365–379 (2021).
Galbiati, A., Verga, L., Giora, E., Zucconi, M. & Ferini-Strambi, L. The risk of neurodegeneration in REM sleep behavior disorder: a systematic review and meta-analysis of longitudinal studies. Sleep Med. Rev. 43, 37–46 (2019).
Kim, J. J. et al. Bidirectional relationship between olfaction and Parkinson’s disease. npj Parkinsons Dis. 10, 232 (2024).
Postuma, R. B. et al. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology 79, 428–434 (2012).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Simuni, T. et al. A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research. Lancet Neurol. 23, 178–190 (2024).
Höglinger, G. U. et al. A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria. Lancet Neurol. 23, 191–204 (2024).
Kalia, L. V. et al. International Parkinson and Movement Disorder Society viewpoint on biological frameworks of Parkinson’s disease: current status and future directions. Mov. Disord. 39, 1710–1715 (2024).
Wüllner, U. et al. The heterogeneity of Parkinson’s disease. J. Neural Transm. 130, 827–838 (2023).
McGlinchey, E. et al. Biomarkers of neurodegeneration across the Global South. Lancet Healthy Longev. 5, 100616 (2024).
Marras, C. et al. Transitioning from subtyping to precision medicine in Parkinson’s disease: a purpose-driven approach. Mov. Disord. 39, 462–471 (2024).
Tanaka, M. Parkinson’s disease: bridging gaps, building biomarkers, and reimagining clinical translation. Cells https://doi.org/10.3390/cells14151161 (2025).
Tanguy, A., Jönsson, L. & Ishihara, L. Inventory of real world data sources in Parkinson’s disease. BMC Neurol. 17, 213 (2017).
Taylor, H. et al. UK Biobank — a unique resource for discovery and translation research on genetics and neurologic disease. Neurol. Genet. 11, e200226 (2025).
Leal, T. P. et al. Genotype–phenotype association study conducted on LARGE-PD reveals novel loci associated with Parkinson’s disease. Preprint at medRxiv https://doi.org/10.1101/2025.07.18.25331793 (2025).
Gottesman, J. et al. Fox Insight at 5 years — a cohort of 54,000 participants contributing longitudinal patient-reported outcome, genetic, and microbiome data relating to Parkinson’s disease. Sci. Data 11, 615 (2024).
Oh, J. H. et al. Whole-genome sequencing reveals an association between small genomic deletions and an increased risk of developing Parkinson’s disease. Exp. Mol. Med. 55, 555–564 (2023).
Lv, J. J., Li, X. Y. & Yang, C. H. Urinary metal levels and their association with Parkinson’s disease risk: insights from NHANES 2013–2020. Front. Public Health 13, 1439325 (2025).
Alvarellos, M. et al. Democratizing clinical-genomic data: how federated platforms can promote benefits sharing in genomics. Front. Genet. 13, 1045450 (2022).
Pandit, M. et al. Prioritizing racial representation in treating people with Parkinson’s disease: an assessment of racial inclusion among Parkinson’s disease clinical trials between 1999–2022. Neurology 100, 2732 (2023).
Schneider, M. G. et al. Minority enrollment in Parkinson’s disease clinical trials. Parkinsonism Relat. Disord. 15, 258–262 (2009).
Di Luca, D. G. et al. Enrollment of participants from marginalized racial and ethnic groups: a comparative assessment of the STEADY-PD III and SURE-PD3 trials. Neurol. Clin. Pract. 13, e200113 (2023).
Tsai, C. C. et al. Representational disparities in the enrollment of Parkinson’s disease clinical trials. Mov. Disord. Clin. Pract. 12, 878–881 (2025).
Moorthy, V. et al. Improving the clinical trial environment and infrastructure: moving from global resolution to action. Lancet Glob. Health 13, e608–e610 (2025).
Vaswani, P. A., Tropea, T. F. & Dahodwala, N. Overcoming barriers to Parkinson disease trial participation: increasing diversity and novel designs for recruitment and retention. Neurotherapeutics 17, 1724–1735 (2020).
Hasson Charles, R. M., Sosa, E., Patel, M. & Erhunmwunsee, L. Health disparities in recruitment and enrollment in research. Thorac. Surg. Clin. 32, 75–82 (2022).
Fox Trial Finder. Parkinson’s Disease. The Michael J. Fox Foundation for Parkinson’s Research https://www.michaeljfox.org/trial-finder (accessed 21 October 2025).
Gerstenberger, J., Bauer, A., Helmschrodt, C., Richter, A. & Richter, F. The novel adaptive rotating beam test unmasks sensorimotor impairments in a transgenic mouse model of Parkinson’s disease. Behav. Brain Res. 304, 102–110 (2016).
Shutinoski, B. et al. Lrrk2 alleles modulate inflammation during microbial infection of mice in a sex-dependent manner. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aas9292 (2019).
Lee, J. et al. Sex-specific neuroprotection by inhibition of the Y-chromosome gene, in experimental Parkinson’s disease. Proc. Natl Acad. Sci. USA 116, 16577–16582 (2019).
Hansen, C. et al. A novel α-synuclein–GFP mouse model displays progressive motor impairment, olfactory dysfunction and accumulation of α-synuclein–GFP. Neurobiol. Dis. 56, 145–155 (2013).
Roshanbin, S. et al. Age-related increase of alpha-synuclein oligomers is associated with motor disturbances in L61 transgenic mice. Neurobiol. Aging 101, 207–220 (2021).
Iwaki, H. et al. Differences in the presentation and progression of Parkinson’s disease by sex. Mov. Disord. 36, 106–117 (2021).
Saul, M. C., Philip, V. M. & Reinholdt, L. G. Center for Systems Neurogenetics of Addiction & Chesler, E. J. High-diversity mouse populations for complex traits. Trends Genet. 35, 501–514 (2019).
Popejoy, A. B. & Fullerton, S. M. Genomics is failing on diversity. Nature 538, 161–164 (2016).
Ghosh, S., Nehme, R. & Barrett, L. E. Greater genetic diversity is needed in human pluripotent stem cell models. Nat. Commun. 13, 7301 (2022).
Bloomfield, G. S. et al. Training and capacity building in LMIC for research in heart and lung diseases: The NHLBI–UnitedHealth Global Health Centers of Excellence program. Glob. Heart 11, 17–25 (2016).
Petersen, O. H. Inequality of research funding between different countries and regions is a serious problem for global science. Function 2, zqab060 (2021).
Marconi, G. A., Teixeira-Dos-Santos, D. & Schuh, A. F. S. Recent advances in the genetics of Parkinson’s disease in underrepresented populations. Curr. Opin. Neurol. 38, 349–354 (2025).
The Edmond J. Safra Fellowship in Movement Disorders. The Michael J. Fox Foundation https://www.michaeljfox.org/edmond-j-safra-fellowship-movement-disorders (accessed 27 September 2025).
Siddiqi, B. & Koemeter-Cox, A. A call to action: promoting diversity, equity, and inclusion in Parkinson’s research and care. J. Parkinsons Dis. 11, 905–908 (2021).
Our commitment to diversity, equity & inclusion. Parkinson’s Foundation https://www.parkinson.org/about-us/vision-mission/diversity-equity-inclusion (accessed 27 September 2025).
Li, S. et al. Cost-effectiveness analysis of insecticide ban aimed at preventing Parkinson’s disease in central California. Sci. Total Environ. 912, 168913 (2024).
Committee on Energy and Commerce. Dr. Emmanuel Bilirakis National Plan to End Parkinson’s Act: Report (to accompany H.R. 2365) (United States Congress House, 2023).
Wasay, M., Grisold, W., Wijeratne, T., Struhal, W. & Younis, S. World Federation of Neurology. The future of brain health advocacy: recommendations of the 2023 World Congress of Neurology advocacy panel. J. Neurol. Sci. 458, 122914 (2024).
Visiting trainee grants. International Parkinson and Movement Disorder Society https://www.movementdisorders.org/MDS/About/MDS-Programs/Visiting-Trainee-Grants.htm (accessed 12 October 2025).
Walker, R. et al. Transforming Parkinson’s Care in Africa (TraPCAf): protocol for a multimethodology National Institute for Health and Care Research Global Health Research Group project. BMC Neurol. 23, 373 (2023).
Fogarty Extramural Programs. Fogarty International Center https://www.fic.nih.gov/Programs (accessed 12 October 2025).
Aamodt, W. W., Willis, A. W. & Dahodwala, N. Racial and ethnic disparities in Parkinson disease: a call to action. Neurol. Clin. Pract. 13, e200138 (2023).
Flanagin, A., Frey, T. & Christiansen, S. L. AMA Manual of Style Committee. Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA 326, 621–627 (2021).
Lin, J. et al. Keeping an eye on Parkinson’s disease: color vision and outer retinal thickness as simple and non-invasive biomarkers. J. Neurol. 272, 351 (2025).
Yu, Q. J. et al. Parkinson disease with constipation: clinical features and relevant factors. Sci. Rep. 8, 567 (2018).
Altundag, A. et al. Cross-culturally modified University of Pennsylvania Smell Identification Test for a Turkish population. Am. J. Rhinol. Allergy 29, e138–e141 (2015).
Bušková, J. et al. Validation of the REM sleep behavior disorder screening questionnaire in the Czech population. BMC Neurol. 19, 110 (2019).
Rivera Mindt, M. et al. The Alzheimer’s Disease Neuroimaging Initiative-4 (ADNI-4) Engagement Core: a culturally informed, community-engaged research (CI-CER) model to advance brain health equity. Alzheimers Dement. 20, 8279–8293 (2024).
Parkinson’s disease and related disorders. Accelerating Medicines Partnership https://amp-pd.org/ (accessed 27 April 2025).
Horvat, L., Horey, D., Romios, P. & Kis-Rigo, J. Cultural competence education for health professionals. Cochrane Database Syst. Rev. 2014, CD009405 (2014).
Skinner, H. G. et al. Using community-based participatory research principles to develop more understandable recruitment and informed consent documents in genomic research. PLoS ONE 10, e0125466 (2015).
May, T. et al. Community-based participatory research and its potential role in supporting diversity in genomic science. J. Health Care Poor Underserved 32, 1208–1224 (2021).
Chahine, L. M. et al. The Black and African American Connections to Parkinson’s Disease (BLAAC PD) study protocol. BMC Neurol. 24, 403 (2024).
Woodland, L., Blignault, I., O’Callaghan, C. & Harris-Roxas, B. A framework for preferred practices in conducting culturally competent health research in a multicultural society. Health Res. Policy Syst. 19, 24 (2021).
Zeissler, M. L. et al. Patient and public involvement and engagement in the development of a platform clinical trial for Parkinson’s disease: an evaluation protocol. J. Parkinsons Dis. 14, 809–821 (2024).
Sanchez, A. V. et al. Designing the fostering inclusivity in research engagement for underrepresented populations in Parkinson’s disease study. Contemp. Clin. Trials 115, 106713 (2022).
Franzen, S. R. P., Chandler, C. & Lang, T. Health research capacity development in low and middle income countries: reality or rhetoric? A systematic meta-narrative review of the qualitative literature. BMJ Open 7, e012332 (2017).
Rizig, M. et al. The International Parkinson Disease Genomics Consortium Africa. Lancet Neurol. 20, 335 (2021).
Kishore, A. et al. Deciphering the genetic architecture of Parkinson’s disease in India. Preprint at medRxiv https://doi.org/10.1101/2025.02.17.25322132 (2025).
Central Asian and Transcaucasian Parkinson’s Disease Consortium & Kaiyrzhanov, R. A biobank for Parkinson’s disease and atypical parkinsonism in central Asian and Transcaucasian regions. Lancet Neurol. 23, 859–860 (2024).
Mohamed, W. et al. The AfrAbia+plus Parkinson’s disease genomic consortium. Lancet Neurol. 23, 140–141 (2024).
Lourenco, M. V., Borelli, W. V., Duran-Aniotz, C., Zimmer, E. R. & de Castro, S. S. Promoting diversity and overcoming publication barriers in Latin American neuroscience and Alzheimer’s disease research: a call to action. Alzheimers Dement. 9, e12378 (2023).
Miyasaki, J. M., Lim, T. T. & Bhidayasiri, R. Editorial: Inclusion, equity, diversity and social justice in movement disorders research. Parkinsonism Relat. Disord. 85, 114–116 (2021).
Hopewell, S. et al. CONSORT 2025 statement: updated guideline for reporting randomised trials. Br. Med. J. 389, e081123 (2025).
Majid, H. et al. Publication dynamics: what can be done to eliminate barriers to publishing full manuscripts by the postgraduate trainees of a low-middle income country? BMC Res. Notes 15, 249 (2022).
Diversity, equity and inclusion in neurology. Nat. Rev. Neurol. 20, 199 (2024).
Amplifying under-represented perspectives. Nat. Commun. 13, 1834 (2022).
Schutte, A. E. & Abdool Karim, Q. Reimagining global hypertension research: from helicopter science to meaningful partnerships. Hypertension 80, 2239–2242 (2023).
Montreal Statement. The World Conferences on Research Integrity Foundation https://www.wcrif.org/guidance/montreal-statement (accessed 3 May 2025).
Global Code of Conduct https://dev.globalcodeofconduct.org/ (accessed 3 May 2025).
Weinreb, C. & Sun, D. S. To dismantle structural racism in science, scientists need to learn how it works. Neuropsychopharmacology 48, 579–582 (2023).
Banaji, M. R., Fiske, S. T. & Massey, D. S. Systemic racism: individuals and interactions, institutions and society. Cogn. Res. Princ. Implic. 6, 82 (2021).
Wonkam, A. et al. “Black Lives Matter and Black Research Matters”: the African Society of Human Genetics’ call to halt racism in science. Mol. Biol. Cell. https://doi.org/10.1091/mbc.E22-04-0122 (2022).
Omodan, B. I. Building reciprocal relationships through decolonial practices in academic research. Cogent Soc. Sci. https://doi.org/10.1080/23311886.2024.2443558 (2025).
Adam, A. et al. Decolonizing global health research: experiences from the women in health and their economic, equity and livelihood statuses during emergency preparedness and response (WHEELER) study. Front. Public Health 13, 1578964 (2025).
Acknowledgements
A.F.S.S. is a research productivity fellow of the Brazilian National Council for Scientific and Technological Development (CNPq) and received support for research from CNPq and the MJFF. D.T.S. received support from the International Parkinson and Movement Disorder Society for a 1 year research fellowship at the Cleveland Clinic.
Author information
Authors and Affiliations
Contributions
D.T.d.S. and A.F.S.S. researched data for the article. All authors contributed substantially to discussion of the content. D.T.d.S. and A.F.S.S. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks Veronica Bruno, Agustín Ibáñez, Norlinah Ibrahim, Nitish Kamble and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teixeira-dos-Santos, D., Tan, AH., Okubadejo, N. et al. Bridging global diversity gaps in Parkinson disease research. Nat Rev Neurol (2026). https://doi.org/10.1038/s41582-026-01183-1
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41582-026-01183-1