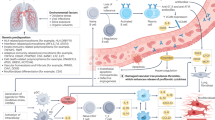
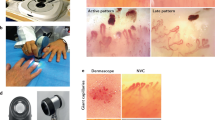
Abstract
Systemic sclerosis (SSc) is a rare autoimmune connective tissue disease with multi-organ involvement, fibrosis and vasculopathy. Treatment in SSc, including early diffuse cutaneous SSc (dcSSc) and the use of organ-specific therapies, has improved, as evident from randomized clinical trials. Treatments for early dcSSc include immunosuppressive agents such as mycophenolate mofetil, methotrexate, cyclophosphamide, rituximab and tocilizumab. Patients with rapidly progressive early dcSSc might be eligible for autologous haematopoietic stem cell transplantation, which can improve survival. Morbidity from interstitial lung disease and pulmonary arterial hypertension is improving with the use of proven therapies. Mycophenolate mofetil has surpassed cyclophosphamide as the initial treatment for SSc-interstitial lung disease. Nintedanib and possibly perfinidone can be considered in SSc pulmonary fibrosis. Pulmonary arterial hypertension is frequently treated with initial combination therapy (for example, with phosphodiesterase 5 inhibitors and endothelin receptor antagonists) and, if necessary, the addition of a prostacyclin analogue. Raynaud phenomenon and digital ulcers are treated with dihydropyridine calcium channel blockers (especially nifedipine), then phosphodiesterase 5 inhibitors or intravenous iloprost. Bosentan can reduce the development of new digital ulcers. Trial data for other manifestations are mostly lacking. Research is needed to develop targeted and highly effective treatments, best practices for organ-specific screening and early intervention, and sensitive outcome measurements.
Key points
-
Treatment of systemic sclerosis (SSc) is organ-based or aimed at disease modification.
-
Autologous haematopoietic stem cell transplantation can improve survival in patients with early diffuse cutaneous SSc who are at high risk of mortality, such as those with very high skin scores (as measured by the modified Rodnan skin score) or moderate skin involvement and worsening interstitial lung disease (ILD).
-
Immunosuppressives and some biologic agents can soften skin and change the natural history of early diffuse cutaneous SSc.
-
Appropriate treatment for patients with early limited cutaneous SSc is unknown, and further research is needed.
-
ILD is usually treated by the use of mycophenolate mofetil as the initial therapy and then other immunosuppressives or biologic agents, but if ILD is fibrotic and progressing, anti-fibrotic therapy can be added, such as nintedanib (and possibly pirfenidone).
-
Raynaud phenomenon in SSc is treated with calcium channel blockers and then phosphodiesterase 5 inhibitors or intravenous iloprost.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Martin Calderon, L. & Pope, J. E. Scleroderma epidemiology update. Curr. Opin. Rheumatol. 33, 122–127 (2021).
LeRoy, E. C. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J. Rheumatol. 15, 202–205 (1988).
Hunzelmann, N. et al. The registry of the German Network for Systemic Scleroderma: frequency of disease subsets and patterns of organ involvement. Rheumatology 47, 1185–1192 (2008).
De Almeida Chaves, S. et al. Sine scleroderma, limited cutaneous, and diffused cutaneous systemic sclerosis survival and predictors of mortality. Arthritis Res. Ther. 23, 295 (2021).
Marangoni, R. G. et al. Systemic sclerosis sine scleroderma: distinct features in a large Brazilian cohort. Rheumatology 52, 1520–1524 (2013).
Koenig, M. et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 58, 3902–3912 (2008).
Bellando-Randone, S. et al. Progression of patients with Raynaud’s phenomenon to systemic sclerosis: a five-year analysis of the European Scleroderma Trial and Research group multicentre, longitudinal registry study for very early diagnosis of systemic sclerosis (VEDOSS). Lancet Rheumatol. 3, e834–e843 (2021).
Muangchan, C. et al. The 15% rule in scleroderma: the frequency of severe organ complications in systemic sclerosis. A systematic review. J. Rheumatol. 40, 1545–1556 (2013).
Akesson, A. & Wollheim, F. A. Organ manifestations in 100 patients with progressive systemic sclerosis: a comparison between the CREST syndrome and diffuse scleroderma. Br. J. Rheumatol. 28, 281–286 (1989).
Thoua, N. M. et al. Assessment of gastrointestinal symptoms in patients with systemic sclerosis in a UK tertiary referral centre. Rheumatology 49, 1770–1775 (2010).
Fretheim, H. et al. Multidimensional tracking of phenotypes and organ involvement in a complete nationwide systemic sclerosis cohort. Rheumatology 59, 2920–2929 (2020).
Steen, V. D. & Medsger, T. A. Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 43, 2437–2444 (2000).
Bussone, G. & Mouthon, L. Interstitial lung disease in systemic sclerosis. Autoimmun. Rev. 10, 248–255 (2011).
Pope, J. E. et al. Systemic sclerosis and associated interstitial lung disease in Ontario, Canada: an examination of prevalence and survival over 10 years. J. Rheumatol. 48, 1427–1434 (2021).
Fernandez-Codina, A. et al. Treatment algorithms for systemic sclerosis according to experts. Arthritis Rheumatol. 70, 1820–1828 (2018).
Khanna, D. et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2, 11–18 (2017).
Herrick, A. L. et al. Patterns and predictors of skin score change in early diffuse systemic sclerosis from the European Scleroderma Observational Study. Ann. Rheum. Dis. 77, 563–570 (2018).
Khanna, D. et al. The American College of Rheumatology provisional composite response index for clinical trials in early diffuse cutaneous systemic sclerosis. Arthritis Care Res. 68, 167–178 (2016).
Spiera, R. et al. Safety and efficacy of lenabasum in a phase II, randomized, placebo-controlled trial in adults with systemic sclerosis. Arthritis Rheumatol. 72, 1350–1360 (2020).
Chung, L. et al. Safety and efficacy of abatacept in early diffuse cutaneous systemic sclerosis (ASSET): open-label extension of a phase 2, double-blind randomised trial. Lancet Rheumatol. 2, e743–e753 (2020).
Khanna, D. et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 387, 2630–2640 (2016).
Khanna, D. et al. Evaluation of American college of rheumatology provisional composite response index in systemic sclerosis (ACR CRISS) in a phase 3 randomized controlled trial [abstract]. Arthritis Rheumatol. 70, 2938 (2018).
Nevskaya, T. et al. Skin improvement is a surrogate for favourable changes in other organ systems in early diffuse cutaneous systemic sclerosis. Rheumatology 59, 1715–1724 (2020).
Zheng, B. et al. Changes in skin score in early diffuse cutaneous systemic sclerosis are associated with changes in global disease severity. Rheumatology 59, 398–406 (2020).
Burt, R. K. et al. Cardiac safe hematopoietic stem cell transplantation for systemic sclerosis with poor cardiac function: a pilot safety study that decreases neutropenic interval to 5 days. Bone Marrow Transpl. 56, 50–59 (2021).
Sullivan, K. M. et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N. Engl. J. Med. 378, 35–47 (2018).
van Laar, J. M. et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 311, 2490–2498 (2014).
Burt, R. K. et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet 378, 498–506 (2011).
Shouval, R. et al. Autologous hematopoietic stem cell transplantation for systemic sclerosis: a systematic review and meta-analysis. Biol. Blood Marrow Transpl. 24, 937–944 (2018).
Maltez, N. et al. Association of autologous hematopoietic stem cell transplantation in systemic sclerosis with marked improvement in health-related quality of life. Arthritis Rheumatol. 73, 305–314 (2021).
van Bijnen, S. et al. Predictive factors for treatment-related mortality and major adverse events after autologous haematopoietic stem cell transplantation for systemic sclerosis: results of a long-term follow-up multicentre study. Ann. Rheum. Dis. 79, 1084–1089 (2020).
Guillaume-Jugnot, P. et al. Autologous haematopoietic stem cell transplantation (AHSCT) in autoimmune disease adult patients in France: analysis of the long-term outcome from the French society for bone marrow transplantation and cellular therapy (SFGM-TC). Clin. Rheumatol. 38, 1501–1511 (2019).
Henes, J. et al. Autologous stem cell transplantation for progressive systemic sclerosis: a prospective non-interventional study from the European Society for Blood and Marrow transplantation autoimmune disease working party. Haematologica 106, 375–383 (2021).
Tashkin, D. P. et al. Cyclophosphamide versus placebo in scleroderma lung disease. N. Engl. J. Med. 354, 2655–2666 (2006).
Tashkin, D. P. et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir. Med. 4, 708–719 (2016).
Pope, J. E. et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 44, 1351–1358 (2001).
van den Hoogen, F. H. et al. Comparison of methotrexate with placebo in the treatment of systemic sclerosis: a 24 week randomized double-blind trial, followed by a 24 week observational trial. Br. J. Rheumatol. 35, 364–372 (1996).
Gordon, J. K. et al. Belimumab for the treatment of early diffuse systemic sclerosis: results of a randomized, double-blind, placebo-controlled, pilot trial. Arthritis Rheumatol. 70, 308–316 (2018).
Khanna, D. et al. Abatacept in early diffuse cutaneous systemic sclerosis: results of a phase II investigator-initiated, multicenter, double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol. 72, 125–136 (2020).
Khanna, D. et al. A phase 2a randomized, double-blind, placebo-controlled study of ziritaxestat in early diffuse cutaneous systemic sclerosis (NOVESA) [abstract]. Arthritis Rheumatol. 72, L09 (2020).
Khanna, D. et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 8, 963–974 (2020).
Khanna, D. et al. Riociguat in patients with early diffuse cutaneous systemic sclerosis (RISE-SSc): randomised, double-blind, placebo-controlled multicentre trial. Ann. Rheum. Dis. 79, 618–625 (2020).
Hudson, M. et al. Cyclophosphamide for the treatment of skin fibrosis in systemic sclerosis: a systematic review [abstract]. Arthritis Rheumatol. 71, 2593 (2019).
Allanore, Y. et al. A randomised, double-blind, placebo-controlled, 24-week, phase II, proof-of-concept study of romilkimab (SAR156597) in early diffuse cutaneous systemic sclerosis. Ann. Rheum. Dis. 79, 1600–1607 (2020).
GlobeNewswire. Corbus Pharmaceuticals announces topline results from RESOLVE-1 phase 3 study of lenabasum for treatment of systemic sclerosis. GlobeNewswire https://www.globenewswire.com/news-release/2020/09/08/2089940/0/en/Corbus-Pharmaceuticals-Announces-Topline-Results-from-RESOLVE-1-Phase-3-Study-of-Lenabasum-for-Treatment-of-Systemic-Sclerosis.html (2020).
Distler, O. et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N. Engl. J. Med. 380, 2518–2528 (2019).
Fernandez-Codina, A., Nevskaya, T. & Pope, J. Brentuximab vedontin for skin involvement in refractory diffuse cutaneous systemic sclerosis, interim results of a phase IIb open-label trial. Ann. Rheum. Dis. 80, 103–104 (2021).
Khanna, D. et al. Tofacitinib in early diffuse cutaneous systemic sclerosis — results of phase I/II investigator-initiated, double-blind randomized placebo-controlled trial [abstract 863]. Arthritis Rheumatol. 71, 1493–1495 (2019).
Karalilova, R. V. et al. Tofacitinib in the treatment of skin and musculoskeletal involvement in patients with systemic sclerosis, evaluated by ultrasound. Rheumatol. Int. 41, 1743–1753 (2021).
Hoa, S. et al. Association between immunosuppressive therapy and course of mild interstitial lung disease in systemic sclerosis. Rheumatology 59, 1108–1117 (2020).
Kowal-Bielecka, O. et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 76, 1327–1339 (2017).
Gernert, M. et al. Immunosuppressive therapy after autologous hematopoietic stem cell transplantation in systemic sclerosis patients-high efficacy of rituximab. Front. Immunol. 12, 817893 (2021).
Gressenberger, P. et al. Rituximab as a treatment option after autologous hematopoietic stem cell transplantation in a patient with systemic sclerosis. J. Pers. Med. 11, 600 (2021).
Arruda, L. C. et al. Resetting the immune response after autologous hematopoietic stem cell transplantation for autoimmune diseases. Curr. Res. Transl. Med. 64, 107–113 (2016).
Nihtyanova, S. I. et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. 66, 1625–1635 (2014).
Steen, V. D. & Medsger, T. A. Changes in causes of death in systemic sclerosis, 1972–2002. Ann. Rheum. Dis. 66, 940–944 (2007).
Elhai, M. et al. Mapping and predicting mortality from systemic sclerosis. Ann. Rheum. Dis. 76, 1897–1905 (2017).
Hoffmann-Vold, A. M. et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann. Rheum. Dis. 80, 219–227 (2021).
Hoffmann-Vold, A. M. et al. POS0063 Progressive interstitial lung disease is frequent also in late disease stages in systemic sclerosis patients from EUSTAR. Ann. Rheum. Dis. 81, 248 (2022).
Nihtyanova, S. I. et al. Using autoantibodies and cutaneous subset to develop outcome-based disease classification in systemic sclerosis. Arthritis Rheumatol. 72, 465–476 (2020).
Savarino, E. et al. Possible connection between gastroesophageal reflux and interstitial pulmonary fibrosis in patients with systemic sclerosis. Recenti Prog. Med. 100, 512–516 (2009).
Zhang, X. J. et al. Association of gastroesophageal factors and worsening of forced vital capacity in systemic sclerosis. J. Rheumatol. 40, 850–858 (2013).
Goh, N. S. et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am. J. Respir. Crit. Care Med. 177, 1248–1254 (2008).
Hoffmann-Vold, A. M. et al. Assessment of recent evidence for the management of patients with systemic sclerosis-associated interstitial lung disease: a systematic review. ERJ Open Res 7, 00235-2020 (2021).
Montesi, S. B. & Caravan, P. Novel imaging approaches in systemic sclerosis-associated interstitial lung disease. Curr. Rheumatol. Rep. 21, 25 (2019).
Goh, N. S. et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol. 69, 1670–1678 (2017).
Volkmann, E. R. et al. Short-term progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann. Rheum. Dis. 78, 122–130 (2019).
Stock, C. J. W. et al. Serum markers of pulmonary epithelial damage in systemic sclerosis-associated interstitial lung disease and disease progression. Respirology 26, 461–468 (2021).
De Lauretis, A. et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J. Rheumatol. 40, 435–446 (2013).
Renaud, L. et al. Prominence of IL6, IGF, TLR, and bioenergetics pathway perturbation in lung tissues of scleroderma patients with pulmonary fibrosis. Front. Immunol. 11, 383 (2020).
Bonhomme, O. et al. Biomarkers in systemic sclerosis-associated interstitial lung disease: review of the literature. Rheumatology 58, 1534–1546 (2019).
Roofeh, D. et al. Management of systemic sclerosis-associated interstitial lung disease. Curr. Opin. Rheumatol. 31, 241–249 (2019).
Bukiri, H. & Volkmann, E. R. Current advances in the treatment of systemic sclerosis. Curr. Opin. Pharmacol. 64, 102211 (2022).
Hoyles, R. K. et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 54, 3962–3970 (2006).
Ebata, S. et al. Safety and efficacy of rituximab for systemic sclerosis: a double-blind, parallel-group comparison, investigators initiated confirmatory randomized clinical trial (DESIRES Study) [abstract]. Arthritis Rheumatol. 73, 0496 (2021).
Hughes, M., Denton, C. P. & Khanna, D. Rituximab for the treatment of systemic sclerosis-interstitial lung disease. Rheumatology 60, 489–491 (2021).
Goswami, R. P. et al. Rituximab in the treatment of systemic sclerosis-related interstitial lung disease: a systematic review and meta-analysis. Rheumatology 60, 557–567 (2021).
Sircar, G. et al. Intravenous cyclophosphamide vs rituximab for the treatment of early diffuse scleroderma lung disease: open label, randomized, controlled trial. Rheumatology 57, 2106–2113 (2018).
Denton, C. P. et al. Therapeutic interleukin-6 blockade reverses transforming growth factor-beta pathway activation in dermal fibroblasts: insights from the faSScinate clinical trial in systemic sclerosis. Ann. Rheum. Dis. 77, 1362–1371 (2018).
Khanna, D. et al. STRATUS: a phase II study of abituzumab in patients with systemic sclerosis-associated interstitial lung disease. J. Rheumatol. 48, 1295–1298 (2021).
Maher, T. M. et al. Rituximab versus intravenous cyclophosphamide in patients with connective tissue disease-associated interstitial lung disease in the UK (RECITAL): a double-blind, double-dummy, randomised, controlled, phase 2b trial. Lancet Respir. Med. 11, 45–54 (2023).
Flaherty, K. R. et al. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 381, 1718–1727 (2019).
Khanna, D. et al. An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS trial. J. Rheumatol. 43, 1672–1679 (2016).
Behr, J. et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 9, 476–486 (2021).
Khanna, D. et al. Combination therapy of mycophenolate mofetil and pirfenidone vs. mycophenolate alone: results from the Scleroderma Lung Study III [abstract]. Arthritis Rheumatol. 74, 0520 (2022).
Perelas, A. et al. Systemic sclerosis-associated interstitial lung disease. Lancet Respir. Med. 8, 304–320 (2020).
Waxman, A. et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N. Engl. J. Med. 384, 325–334 (2021).
Hoffmann-Vold, A. M. et al. The need for a holistic approach for SSc-ILD — achievements and ambiguity in a devastating disease. Respir. Res. 21, 197 (2020).
Bernstein, E. J. et al. Survival of adults with systemic sclerosis following lung transplantation: a nationwide cohort study. Arthritis Rheumatol. 67, 1314–1322 (2015).
Crespo, M. M. et al. Lung transplant in patients with scleroderma compared with pulmonary fibrosis. Short- and long-term outcomes. Ann. Am. Thorac. Soc. 13, 84–92 (2016).
Kreuter, M. et al. Impact of lung function decline on time to hospitalisation events in systemic sclerosis-associated interstitial lung disease (SSc-ILD): a joint model analysis. Arthritis Res. Ther. 24, 19 (2022).
Hoa, S. et al. Association between immunosuppressive therapy and incident risk of interstitial lung disease in systemic sclerosis. Chest 160, 2158–2162 (2021).
Naidu, G. S. R. S. N. K. et al. Effect of mycophenolate mofetil (MMF) on systemic sclerosis-related interstitial lung disease with mildly impaired lung function: a double-blind, placebo-controlled, randomized trial. Rheumatol. Int. 40, 207–216 (2020).
Yomono, K. & Kuwana, M. Outcomes in patients with systemic sclerosis undergoing early vs delayed intervention with potential disease-modifying therapies. Rheumatology 61, 3677–3685 (2022).
Vonk, M. C., Vandecasteele, E. & van Dijk, A. P. Pulmonary hypertension in connective tissue diseases, new evidence and challenges. Eur. J. Clin. Invest. 51, e13453 (2021).
Morrisroe, K. et al. Epidemiology and disease characteristics of systemic sclerosis-related pulmonary arterial hypertension: results from a real-life screening programme. Arthritis Res. Ther. 19, 42 (2017).
Simonneau, G. et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 53, 1801913 (2019).
Jiang, Y., Turk, M. A. & Pope, J. E. Factors associated with pulmonary arterial hypertension (PAH) in systemic sclerosis (SSc). Autoimmun. Rev. 19, 102602 (2020).
Connolly, M. J. et al. Prognostic significance of computed tomography criteria for pulmonary veno-occlusive disease in systemic sclerosis-pulmonary arterial hypertension. Rheumatology 56, 2197–2203 (2017).
Johnson, S. R. et al. Venous thromboembolism in systemic sclerosis: prevalence, risk factors, and effect on survival. J. Rheumatol. 45, 942–946 (2018).
Ende-Verhaar, Y. M. et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur. Respir. J. 49, 1601792 (2017).
Weatherald, J. et al. Screening for pulmonary arterial hypertension in systemic sclerosis. Eur. Respir. Rev. 28, 190023 (2019).
Lee, P. et al. Mortality in systemic sclerosis (scleroderma). Q. J. Med. 82, 139–148 (1992).
Johnson, S. R. et al. Pulmonary veno-occlusive disease and scleroderma associated pulmonary hypertension. J. Rheumatol. 33, 2347–2350 (2006).
Galie, N. et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 37, 67–119 (2016).
Johnson, S. R., Granton, J. T. & Mehta, S. Thrombotic arteriopathy and anticoagulation in pulmonary hypertension. Chest 130, 545–552 (2006).
Johnson, S. R., Mehta, S. & Granton, J. T. Anticoagulation in pulmonary arterial hypertension: a qualitative systematic review. Eur. Respir. J. 28, 999–1004 (2006).
Lei, Y. et al. The effects of oral treatment for systemic sclerosis related pulmonary arterial hypertension: a systematic review and meta-analysis. Mod. Rheumatol. 31, 151–161 (2021).
Hitzerd, E. et al. Endothelin receptor antagonism during preeclampsia: a matter of timing? Clin. Sci. 133, 1341–1352 (2019).
Humbert, M. et al. Riociguat for the treatment of pulmonary arterial hypertension associated with connective tissue disease: results from PATENT-1 and PATENT-2. Ann. Rheum. Dis. 76, 422–426 (2017).
Galie, N. et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 53, 1801889 (2019).
Galie, N. et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N. Engl. J. Med. 373, 834–844 (2015).
Belge, C. & Delcroix, M. Treatment of pulmonary arterial hypertension with the dual endothelin receptor antagonist macitentan: clinical evidence and experience. Ther. Adv. Respir. Dis. 13, 1753466618823440 (2019).
Coghlan, J. G. et al. Initial combination therapy with ambrisentan and tadalafil in connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH): subgroup analysis from the AMBITION trial. Ann. Rheum. Dis. 76, 1219–1227 (2017).
Paul, G. A. et al. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br. J. Clin. Pharmacol. 60, 107–112 (2005).
Zamanian, R. T. et al. Safety and efficacy of B-cell depletion with rituximab for the treatment of systemic sclerosis-associated pulmonary arterial hypertension: a multicenter, double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 204, 209–221 (2021).
Meier, F. M. et al. Update on the profile of the EUSTAR cohort: an analysis of the EULAR Scleroderma Trials and Research group database. Ann. Rheum. Dis. 71, 1355–1360 (2012).
Hughes, M. & Herrick, A. L. Digital ulcers in systemic sclerosis. Rheumatology 56, 14–25 (2017).
Khimdas, S. et al. Associations with digital ulcers in a large cohort of systemic sclerosis: results from the Canadian Scleroderma Research Group registry. Arthritis Care Res. 63, 142–149 (2011).
Merkel, P. A. et al. Measuring disease activity and functional status in patients with scleroderma and Raynaud’s phenomenon. Arthritis Rheum. 46, 2410–2420 (2002).
Hughes, M. et al. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat. Rev. Rheumatol. 16, 208–221 (2020).
Pauling, J. D. The challenge of establishing treatment efficacy for cutaneous vascular manifestations of systemic sclerosis. Expert. Rev. Clin. Immunol. 14, 431–442 (2018).
Denton, C. P. et al. BSR and BHPR guidelines for the treatment of systemic sclerosis. Rheumatology 55, 1906–1910 (2016).
University of Oxford. Oxford Centre for Evidence-Based Medicine: levels of evidence (March 2009). CEBM https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (2009).
Fernández-Codina, A., Cañas-Ruano, E. & Pope, J. E. Management of Raynaud’s phenomenon in systemic sclerosis — a practical approach. J. Scleroderma Relat. Disord. 4, 102–110 (2019).
Rirash, F. et al. Calcium channel blockers for primary and secondary Raynaud’s phenomenon. Cochrane Database Syst. Rev. 12, CD000467 (2017).
Roustit, M. et al. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud’s phenomenon: systematic review and meta-analysis of randomised trials. Ann. Rheum. Dis. 72, 1696–1699 (2013).
Pope, J. et al. Iloprost and cisaprost for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database Syst. Rev. 1998, CD000953 (2000).
Ingegnoli, F. et al. Practical suggestions on intravenous iloprost in Raynaud’s phenomenon and digital ulcer secondary to systemic sclerosis: systematic literature review and expert consensus. Semin. Arthritis Rheum. 48, 686–693 (2019).
Marasini, B. et al. Comparison between iloprost and alprostadil in the treatment of Raynaud’s phenomenon. Scand. J. Rheumatol. 33, 253–256 (2004).
Curtiss, P. et al. A systematic review and meta-analysis of the effects of topical nitrates in the treatment of primary and secondary Raynaud’s phenomenon. J. Am. Acad. Dermatol. 78, 1110–1118.e3 (2018).
Dziadzio, M. et al. Losartan therapy for Raynaud’s phenomenon and scleroderma: clinical and biochemical findings in a fifteen-week, randomized, parallel-group, controlled trial. Arthritis Rheum. 42, 2646–2655 (1999).
van der Meer, J. et al. A double-blind controlled trial of low dose acetylsalicylic acid and dipyridamole in the treatment of Raynaud’s phenomenon. VASA Suppl. 18, 71–75 (1987).
Abou-Raya, A., Abou-Raya, S. & Helmii, M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J. Rheumatol. 35, 1801–1808 (2008).
Coleiro, B. et al. Treatment of Raynaud’s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology 40, 1038–1043 (2001).
Wasserman, A. & Brahn, E. Systemic sclerosis: bilateral improvement of Raynaud’s phenomenon with unilateral digital sympathectomy. Semin. Arthritis Rheum. 40, 137–146 (2010).
Bank, J. et al. Fat grafting to the hand in patients with Raynaud phenomenon: a novel therapeutic modality. Plast. Reconstr. Surg. 133, 1109–1118 (2014).
Motegi, S. I. et al. Efficacy of botulinum toxin B injection for Raynaud’s phenomenon and digital ulcers in patients with systemic sclerosis. Acta Derm. Venereol. 97, 843–850 (2017).
Bello, R. J. et al. The therapeutic efficacy of botulinum toxin in treating scleroderma-associated Raynaud’s phenomenon: a randomized, double-blind, placebo-controlled clinical trial. Arthritis Rheumatol. 69, 1661–1669 (2017).
Tingey, T. et al. Meta-analysis of healing and prevention of digital ulcers in systemic sclerosis. Arthritis Care Res. 65, 1460–1471 (2013).
Shenoy, P. D. et al. Efficacy of tadalafil in secondary Raynaud’s phenomenon resistant to vasodilator therapy: a double-blind randomized cross-over trial. Rheumatology 49, 2420–2428 (2010).
Hachulla, E. et al. Efficacy of sildenafil on ischaemic digital ulcer healing in systemic sclerosis: the placebo-controlled SEDUCE study. Ann. Rheum. Dis. 75, 1009–1015 (2016).
Matucci-Cerinic, M. et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 70, 32–38 (2011).
Del Papa, N. et al. Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: results of a monocentric randomized controlled study. Arthritis Res. Ther. 21, 7 (2019).
Momeni, A. et al. Surgical treatment of systemic sclerosis — is it justified to offer peripheral sympathectomy earlier in the disease process? Microsurgery 35, 441–446 (2015).
Allanore, Y. et al. Clinical characteristics and predictors of gangrene in patients with systemic sclerosis and digital ulcers in the Digital Ulcer Outcome Registry: a prospective, observational cohort. Ann. Rheum. Dis. 75, 1736–1740 (2016).
Hughes, M. et al. Consensus best practice pathway of the UK Scleroderma Study Group: digital vasculopathy in systemic sclerosis. Rheumatology 54, 2015–2024 (2015).
Malenfant, D., Catton, M. & Pope, J. E. The efficacy of complementary and alternative medicine in the treatment of Raynaud’s phenomenon: a literature review and meta-analysis. Rheumatology 48, 791–795 (2009).
Benza, R. L. et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 141, 354–362 (2012).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
J.E.P. declares that she has research grants from AbbVie, Bayer, BI, BMS, Frensenius Kabi, Lilly, Mallinckrodt Pharmaceuticals, Merck, Roche, Seattle Genetics; that she has consulted for AbbVie, Amgen, BI, BMS, Celltrion, EMERALD, Frensenius Kabi, Galapagos, Gilead, Janssen, Lilly, Mallinckrodt Pharmaceuticals, Medexus, Merck, Mitsubishi Tanabe Pharma, Novartis, Pfizer, Roche, Sandoz, Samsung, Sanofi, Sobi, Teva, Viatris; and that she has been a speaker or attended an advisory board for AbbVie, Amgen, BI, BMS, Frensenius Kabi, Galapagos, Gilead, Janssen, Lilly, Merck, Novartis, Pfizer, Sandoz, Sanofi, UCB. C.P.D. declares that he has received consultancy or speaker fees from GlaxoSmithKline, Roche, Boehringer Ingelheim, Sanofi-Aventis, Galapagos, Inventiva, Corbus, Acceleron, Horizon, Gesynta; and that he has received research grants to his institution from GlaxoSmithKline, ARXX Therapeutics, Servier and Horizon Therapeutics. S.R.J. declares that he has been a site investigator for clinical trials sponsored by Bayer, Boehringer Ingelheim, Corbus, GlaxoSmithKline; that he has served on advisory boards for Boehringer Ingelheim, Corbus and Ikaria; and that he has been supported by the Oscar and Eleonor Markovitz Scleroderma Research Fund and the Gurmej Kaur Dhanda Scleroderma Research Fund. A.F.-C. declares that he has received grant support from the Scleroderma Society of Ontario and honoraria from Actelion, Bayer, Boehringer-Ingelheim. M.H. declares that she has received research grants from Boehringer Ingelheim and Bristol Myers Squibb and that she has participated in advisory boards for Boehringer Ingelheim, Alexion and Mallinckrodt. T.N. declares no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks A. Balbir-Gurman, J. Pauling, E Volkmann and M. Vonk for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pope, J.E., Denton, C.P., Johnson, S.R. et al. State-of-the-art evidence in the treatment of systemic sclerosis. Nat Rev Rheumatol 19, 212–226 (2023). https://doi.org/10.1038/s41584-023-00909-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41584-023-00909-5
This article is cited by
-
Thiazolidine derivative (LPSF/CR-35) modulates the production of IL-17A, IL-10, IL-4, TFN, CCL2 and CXCL8 in PBMC from systemic sclerosis patients
Pharmacological Reports (2026)
-
Spatial transcriptomics in autoimmune rheumatic disease: potential clinical applications and perspectives
Inflammation and Regeneration (2025)
-
Efficacy and safety of mesenchymal stromal cell transplantation in the treatment of autoimmune and rheumatic immune diseases: a systematic review and meta-analysis of randomized controlled trials
Stem Cell Research & Therapy (2025)
-
Analysis of related deaths in patients with systemic sclerosis combined with renal failure in the United States from 1999 to 2020
Scientific Reports (2025)
-
Emerging therapies for the treatment of systemic sclerosis
Nature Reviews Rheumatology (2025)