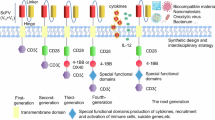
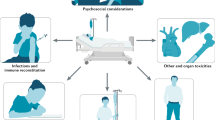
Abstract
Initial success with B cell-targeted chimeric antigen receptor (CAR) T cells for the treatment of systemic lupus erythematosus and other rheumatic diseases has generated enthusiasm for the broad application of this technology outside of the field of oncology. Paediatric patients with severe rheumatic diseases require lifelong therapy with a substantial toxicity burden and a high cost of care. Paradigm-shifting treatments, including CAR T cells, are desperately needed. Although CAR T cell therapy shows promise for paediatric rheumatic diseases, there are unique aspects of care compared with adults, which require careful consideration and expertise. In response, we established the Integrated Multidisciplinary Paediatric Autoimmunity and Cell Therapy (IMPACT) working group, comprising international experts in the fields of paediatric rheumatology, oncology and cellular therapy, immunology and nephrology, to address the challenges of introducing cell therapies to patients with paediatric-onset autoimmune diseases. Given the possible benefits, we advocate for the study of CAR T cells in paediatric patients with rheumatic diseases who carry a lifelong risk of morbidity and mortality from chronic illness and medication toxicity. As this patient population is relatively small, consensus around definitions of success, robust study of predictors of response and uniform assessment and reporting of toxicities are critical to advancing the field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pasquini, M. C. et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 4, 5414–5424 (2020).
Boardman, A. P. & Salles, G. CAR T-cell therapy in large B cell lymphoma. Hematol. Oncol. 41, 112–118 (2023).
Gardner, R. A. et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129, 3322–3331 (2017).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Haghikia, A. et al. Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 22, 1104–1105 (2023).
Auth, J. et al. CD19-targeting CAR T-cell therapy in patients with diffuse systemic sclerosis: a case series. Lancet Rheumatol. 7, e83–e93 (2024).
Qin, C. et al. Single-cell analysis of refractory anti-SRP necrotizing myopathy treated with anti-BCMA CAR-T cell therapy. Proc. Natl Acad. Sci. USA 121, e2315990121 (2024).
Lodka, D. et al. CD19-targeting CAR T cells protect from ANCA-induced acute kidney injury. Ann. Rheum. Dis. 83, 499–507 (2024).
Lee, J. et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J. Clin. Invest. 130, 6317–6324 (2020).
Sadun, R. E. & Foster, M. H. Deja vu but new: using T cells to deplete B cells to treat lupus. Am. J. Kidney Dis. 74, 708–710 (2019).
Kansal, R. et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med. 11, eaav1648 (2019).
Muller, F. et al. CD19 CAR T-cell therapy in autoimmune disease — a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024).
Wilkinson, M. G. L. & Rosser, E. C. B cells as a therapeutic target in paediatric rheumatic disease. Front. Immunol. 10, 214 (2019).
Liu, T. et al. Spatial transcriptomics identifies cellular and molecular characteristics of scleroderma skin lesions: pilot study in juvenile scleroderma. Int. J. Mol. Sci. 25, 9182 (2024).
Torok, K. S. Updates in systemic sclerosis treatment and applicability to pediatric scleroderma. Rheum. Dis. Clin. North. Am. 47, 757–780 (2021).
Bloom, J. L. & Wu, E. Y. Update on antineutrophil cytoplasmic autoantibody vasculitis in children. Curr. Opin. Rheumatol. 36, 336–343 (2024).
Mahmoud, I. et al. Efficacy and safety of rituximab in the management of pediatric systemic lupus erythematosus: a systematic review. J. Pediatr. 187, 213–219.e2 (2017).
Balevic, S. J. et al. Extrapolation of adult efficacy data to pediatric systemic lupus erythematosus: evaluating similarities in exposure-response. J. Clin. Pharmacol. 63, 105–118 (2023).
Sherman, M. A. et al. Treatment escalation patterns to start biologics in refractory moderate juvenile dermatomyositis among members of the Childhood Arthritis and Rheumatology Research Alliance. Pediatr. Rheumatol. Online J. 21, 3 (2023).
Oddis, C. V. et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 65, 314–324 (2013).
Aggarwal, R. et al. Predictors of clinical improvement in rituximab-treated refractory adult and juvenile dermatomyositis and adult polymyositis. Arthritis Rheumatol. 66, 740–749 (2014).
Gagne, S. J. et al. Comparing rituximab and cyclophosphamide in induction therapy for childhood-onset anti-neutrophil cytoplasmic antibody-associated vasculitis: an ARChiVe registry cohort study. Arthritis Care Res. 77, 504–512 (2025).
Jamois, C. et al. Rituximab pediatric drug development: pharmacokinetic and pharmacodynamic modeling to inform regulatory approval for rituximab treatment in patients with granulomatosis with polyangiitis or microscopic polyangiitis. Clin. Transl. Sci. 15, 2172–2183 (2022).
Md Yusof, M. Y. et al. Predicting and managing primary and secondary non-response to rituximab using B-cell biomarkers in systemic lupus erythematosus. Ann. Rheum. Dis. 76, 1829–1836 (2017).
Wobma, H., Chang, J. C. & Prockop, S. E. Releasing our model T — chimeric antigen receptor (CAR) T-cells for autoimmune indications. Curr. Opin. Rheumatol. 37, 128–135 (2025).
Tur, C. et al. CD19-CAR T-cell therapy induces deep tissue depletion of B cells. Ann. Rheum. Dis. 84, 106–114 (2025).
Krickau, T. et al. CAR T-cell therapy rescues adolescent with rapidly progressive lupus nephritis from haemodialysis. Lancet 403, 1627–1630 (2024).
He, X. et al. Treatment of two pediatric patients with refractory systemic lupus erythematosus using CD19-targeted CAR T-cells. Autoimmun. Rev. 24, 103692 (2025).
Nicolai, R. et al. Autologous CD19-targeting CAR T cells in a patient with refractory juvenile dermatomyositis. Arthritis Rheumatol. 76, 1560–1565 (2024).
Schett, G. et al. Advancements and challenges in CAR T cell therapy in autoimmune diseases. Nat. Rev. Rheumatol. 20, 531–544 (2024).
Chung, J. B., Brudno, J. N., Borie, D. & Kochenderfer, J. N. Chimeric antigen receptor T cell therapy for autoimmune disease. Nat. Rev. Immunol. 24, 830–845 (2024).
Stojkic, I. et al. CAR T cell therapy for refractory pediatric systemic lupus erythematosus: a new era of hope? Pediatr. Rheumatol. Online J. 22, 72 (2024).
Bernatsky, S. et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 54, 2550–2557 (2006).
Ambrose, N. et al. Differences in disease phenotype and severity in SLE across age groups. Lupus 25, 1542–1550 (2016).
Brunner, H. I., Gladman, D. D., Ibanez, D., Urowitz, M. D. & Silverman, E. D. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum. 58, 556–562 (2008).
Hersh, A. O. et al. Differences in long-term disease activity and treatment of adult patients with childhood- and adult-onset systemic lupus erythematosus. Arthritis Rheum. 61, 13–20 (2009).
Tucker, L. B. et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus 17, 314–322 (2008).
Robinson, A. B. & Reed, A. M. Clinical features, pathogenesis and treatment of juvenile and adult dermatomyositis. Nat. Rev. Rheumatol. 7, 664–675 (2011).
Papadopoulou, C. & McCann, L. J. The vasculopathy of juvenile dermatomyositis. Front. Pediatr. 6, 284 (2018).
Boros, C. et al. Juvenile dermatomyositis: what comes next? Long-term outcomes in childhood myositis from a patient perspective. Pediatr. Rheumatol. Online J. 20, 102 (2022).
Iudici, M. et al. Childhood- versus adult-onset ANCA-associated vasculitides: a nested, matched case-control study from the French vasculitis study group registry. Autoimmun. Rev. 17, 108–114 (2018).
Foeldvari, I. et al. Are diffuse and limited juvenile systemic sclerosis different in clinical presentation? Clinical characteristics of a juvenile systemic sclerosis cohort. J. Scleroderma Relat. Disord. 4, 49–61 (2019).
Foeldvari, I. et al. Differences sustained between diffuse and limited forms of juvenile systemic sclerosis in an expanded international cohort. Arthritis Care Res. 74, 1575–1584 (2022).
Stevens, B. E. et al. Clinical characteristics and factors associated with disability and impaired quality of life in children with juvenile systemic sclerosis: results from the Childhood Arthritis and Rheumatology Research Alliance legacy registry. Arthritis Care Res. 70, 1806–1813 (2018).
Mirguet, A. et al. Long-term outcomes of childhood-onset systemic lupus erythematosus. Rheumatology 64, 2209–2213 (2024).
Bitencourt, N. et al. “You just have to keep going, you can’t give up”: coping mechanisms among young adults with lupus transferring to adult care. Lupus 30, 2221–2229 (2021).
Tsaltskan, V. et al. Long-term outcomes in juvenile myositis patients. Semin. Arthritis Rheum. 50, 149–155 (2020).
Sanner, H. et al. Long-term muscular outcome and predisposing and prognostic factors in juvenile dermatomyositis: a case-control study. Arthritis Care Res. 62, 1103–1111 (2010).
Foeldvari, I., Nihtyanova, S. I., Wierk, A. & Denton, C. P. Characteristics of patients with juvenile onset systemic sclerosis in an adult single-center cohort. J. Rheumatol. 37, 2422–2426 (2010).
Arulkumaran, N. et al. Long- term outcome of paediatric patients with ANCA vasculitis. Pediatr. Rheumatol. Online J. 9, 12 (2011).
Hiraki, L. T. et al. End-stage renal disease due to lupus nephritis among children in the US, 1995–2006. Arthritis Rheum. 63, 1988–1997 (2011).
Ward, L. M. et al. Osteoporotic fractures and vertebral body reshaping in children with glucocorticoid-treated rheumatic disorders. J. Clin. Endocrinol. Metab. 106, e5195–e5207 (2021).
Silva, C. A. & Brunner, H. I. Gonadal functioning and preservation of reproductive fitness with juvenile systemic lupus erythematosus. Lupus 16, 593–599 (2007).
Silva, M. F. et al. A multicenter study of invasive fungal infections in patients with childhood-onset systemic lupus erythematosus. J. Rheumatol. 42, 2296–2303 (2015).
Schanberg, L. E. et al. Premature atherosclerosis in pediatric systemic lupus erythematosus: risk factors for increased carotid intima-media thickness in the atherosclerosis prevention in pediatric lupus erythematosus cohort. Arthritis Rheum. 60, 1496–1507 (2009).
Sabbagh, S. E. et al. Risk factors associated with Pneumocystis jirovecii pneumonia in juvenile myositis in North America. Rheumatology 60, 829–836 (2021).
Fisler, R. E., Liang, M. G., Fuhlbrigge, R. C., Yalcindag, A. & Sundel, R. P. Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis. J. Am. Acad. Dermatol. 47, 505–511 (2002).
Yang, Y., Kumar, S., Lim, L. S., Silverman, E. D. & Levy, D. M. Risk factors for symptomatic avascular necrosis in childhood-onset systemic lupus erythematosus. J. Rheumatol. 42, 2304–2309 (2015).
Ren, Z., Laumann, A. E. & Silverberg, J. I. Association of dermatomyositis with systemic and opportunistic infections in the United States. Arch. Dermatol. Res. 311, 377–387 (2019).
Restrepo-Escobar, M., N, A. R., Hernandez-Zapata, L. J., Velasquez, M. & Eraso, R. Factors associated with infection amongst paediatric patients with systemic lupus erythematosus treated in the intensive care unit. Lupus 28, 1141–1147 (2019).
Ardalan, K., Lloyd-Jones, D. M. & Schanberg, L. E. Cardiovascular health in pediatric rheumatologic diseases. Rheum. Dis. Clin. North. Am. 48, 157–181 (2022).
Nordal, E. et al. Growth and puberty in juvenile dermatomyositis: a longitudinal cohort study. Arthritis Care Res. 72, 265–273 (2020).
Shiff, N. J. et al. Glucocorticoid-related changes in body mass index among children and adolescents with rheumatic diseases. Arthritis Care Res. 65, 113–121 (2013).
Santiago, R. A., Silva, C. A., Caparbo, V. F., Sallum, A. M. & Pereira, R. M. Bone mineral apparent density in juvenile dermatomyositis: the role of lean body mass and glucocorticoid use. Scand. J. Rheumatol. 37, 40–47 (2008).
Khojah, A., Liu, V., Morgan, G., Shore, R. M. & Pachman, L. M. Changes in total body fat and body mass index among children with juvenile dermatomyositis treated with high-dose glucocorticoids. Pediatr. Rheumatol. Online J. 19, 118 (2021).
Knight, A. M., Trupin, L., Katz, P., Yelin, E. & Lawson, E. F. Depression risk in young adults with juvenile- and adult-onset lupus: twelve years of followup. Arthritis Care Res. 70, 475–480 (2018).
Livermore, P. et al. Being on the juvenile dermatomyositis rollercoaster: a qualitative study. Pediatr. Rheumatol. Online J. 17, 30 (2019).
Ardalan, K. et al. Parent perspectives on addressing emotional health for children and young adults with juvenile myositis. Arthritis Care Res. 73, 18–29 (2021).
Fawole, O. A. et al. Engaging patients and parents to improve mental health intervention for youth with rheumatological disease. Pediatr. Rheumatol. Online J. 19, 19 (2021).
Treemarcki, E. B., Danguecan, A. N., Cunningham, N. R. & Knight, A. M. Mental health in pediatric rheumatology: an opportunity to improve outcomes. Rheum. Dis. Clin. North. Am. 48, 67–90 (2022).
Donnelly, C. et al. Fatigue and depression predict reduced health-related quality of life in childhood-onset lupus. Lupus 27, 124–133 (2018).
Ruperto, N. et al. Health-related quality of life in juvenile-onset systemic lupus erythematosus and its relationship to disease activity and damage. Arthritis Rheum. 51, 458–464 (2004).
Moorthy, L. N. et al. Relationship between health-related quality of life, disease activity and disease damage in a prospective international multicenter cohort of childhood onset systemic lupus erythematosus patients. Lupus 26, 255–265 (2017).
Apaz, M. T. et al. Health-related quality of life of patients with juvenile dermatomyositis: results from the Pediatric Rheumatology International Trials Organisation multinational quality of life cohort study. Arthritis Rheum. 61, 509–517 (2009).
Tollisen, A., Sanner, H., Flato, B. & Wahl, A. K. Quality of life in adults with juvenile-onset dermatomyositis: a case-control study. Arthritis Care Res. 64, 1020–1027 (2012).
Neely, J. et al. Baseline characteristics of children with juvenile dermatomyositis enrolled in the first year of the new Childhood Arthritis and Rheumatology Research Alliance registry. Pediatr. Rheumatol. Online J. 20, 50 (2022).
Groot, N. et al. Effects of childhood-onset systemic lupus erythematosus on academic achievements and employment in adult life. J. Rheumatol. 48, 915–923 (2021).
Zelko, F. et al. Academic outcomes in childhood-onset systemic lupus erythematosus. Arthritis Care Res. 64, 1167–1174 (2012).
Lim, L. S. H. et al. A population-based study of grade 12 academic performance in adolescents with childhood-onset chronic rheumatic diseases. J. Rheumatol. 49, 299–306 (2022).
Moorthy, L. N., Peterson, M. G., Hassett, A., Baratelli, M. & Lehman, T. J. Impact of lupus on school attendance and performance. Lupus 19, 620–627 (2010).
Brunner, H. I., Sherrard, T. M. & Klein-Gitelman, M. S. Cost of treatment of childhood-onset systemic lupus erythematosus. Arthritis Rheum. 55, 184–188 (2006).
Kwa, M. C., Silverberg, J. I. & Ardalan, K. Inpatient burden of juvenile dermatomyositis among children in the United States. Pediatr. Rheumatol. Online J. 16, 70 (2018).
Maus, M. V. & Nikiforow, S. The why, what, and how of the new FACT standards for immune effector cells. J. Immunother. Cancer 5, 36 (2017).
Food and Drug Administration. Long term follow-up after administration of human gene therapy products — guidance for industry https://www.fda.gov/regulatory-information/search-fda-guidance-documents/long-term-follow-after-administration-human-gene-therapy-products (2020).
Fern, L. A. et al. Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials. Lancet Oncol. 15, e341–e350 (2014).
Correll, C. K. et al. 2015 American College of Rheumatology workforce study and demand projections of pediatric rheumatology workforce, 2015-2030. Arthritis Care Res. 74, 340–348 (2022).
Orr, C. J. et al. Projecting the future pediatric subspecialty workforce: summary and recommendations. Pediatrics 153, e2023063678T (2024).
Hall, A. G. et al. Access to chimeric antigen receptor T cell clinical trials in underrepresented populations: a multicenter cohort study of pediatric and young adult acute lymphoblastic leukemia patients. Transpl. Cell Ther. 29, 356.e1–356.e7 (2023).
Auletta, J. J. et al. Assessing Medicaid coverage for hematopoietic cell transplantation and chimeric antigen receptor T cell therapy: a project from the American Society for Transplantation and Cellular Therapy and the National Marrow Donor Program ACCESS initiative. Transpl. Cell Ther. 29, 713–720 (2023).
Steineck, A. et al. Access to CARe: a narrative of real-world medical decision-making to access chimeric antigen receptor (CAR) T-cell therapy in children, adolescents, and young adults. Pediatr. Blood Cancer 72, e31516 (2025).
Sainatham, C. et al. The current socioeconomic and regulatory landscape of immune effector cell therapies. Front. Med. 11, 1462307 (2024).
Islam, N., Budvytyte, L., Khera, N. & Hilal, T. Disparities in clinical trial enrollment — focus on CAR-T and bispecific antibody therapies. Curr. Hematol. Malig. Rep. 20, 1 (2024).
Newman, H. et al. Impact of poverty and neighborhood opportunity on outcomes for children treated with CD19-directed CAR T-cell therapy. Blood 141, 609–619 (2023).
Ahmed, N. et al. Socioeconomic and racial disparity in chimeric antigen receptor T cell therapy access. Transpl. Cell Ther. 28, 358–364 (2022).
Karmali, R. et al. Impact of race and social determinants of health on outcomes in patients with aggressive B-cell NHL treated with CAR-T therapy. Blood Adv. 8, 2592–2599 (2024).
Sureda, A. et al. Logistical challenges of CAR T-cell therapy in non-Hodgkin lymphoma: a survey of healthcare professionals. Future Oncol. 20, 2855–2868 (2024).
Vara, E., Gilbert, M. & Ruth, N. M. Health disparities in outcomes of pediatric systemic lupus erythematosus. Front. Pediatr. 10, 879208 (2022).
Akinsete, A. M., Woo, J. M. P. & Rubinstein, T. B. Disparities in pediatric rheumatic diseases. Rheum. Dis. Clin. North. Am. 48, 183–198 (2022).
Rubinstein, T. B. & Knight, A. M. Disparities in childhood-onset lupus. Rheum. Dis. Clin. North Am. 46, 661–672 (2020).
Phillippi, K. et al. Race, income, and disease outcomes in juvenile dermatomyositis. J. Pediatr. 184, 38–44.e1 (2017).
Deng, Y. & Tsao, B. P. Updates in lupus genetics. Curr. Rheumatol. Rep. 19, 68 (2017).
Qin, Y., Ma, J. & Vinuesa, C. G. Monogenic lupus: insights into disease pathogenesis and therapeutic opportunities. Curr. Opin. Rheumatol. 36, 191–200 (2024).
Costa-Reis, P. & Sullivan, K. E. Monogenic lupus: it’s all new! Curr. Opin. Immunol. 49, 87–95 (2017).
Demirkaya, E., Sahin, S., Romano, M., Zhou, Q. & Aksentijevich, I. New horizons in the genetic etiology of systemic lupus erythematosus and lupus-like disease: monogenic lupus and beyond. J. Clin. Med. 9, 712 (2020).
Webb, R. et al. Early disease onset is predicted by a higher genetic risk for lupus and is associated with a more severe phenotype in lupus patients. Ann. Rheum. Dis. 70, 151–156 (2011).
Tirosh, I. et al. Whole exome sequencing in childhood-onset lupus frequently detects single gene etiologies. Pediatr. Rheumatol. Online J. 17, 52 (2019).
Misztal, M. C. et al. Genome-wide sequencing identified rare genetic variants for childhood-onset monogenic lupus. J. Rheumatol. 50, 671–675 (2023).
Belot, A. et al. Contribution of rare and predicted pathogenic gene variants to childhood-onset lupus: a large, genetic panel analysis of British and French cohorts. Lancet Rheumatol. 2, e99–e109 (2020).
Langefeld, C. D. et al. Transancestral mapping and genetic load in systemic lupus erythematosus. Nat. Commun. 8, 16021 (2017).
Mougiakakos, D. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 385, 567–569 (2021).
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28, 2124–2132 (2022).
Chellapandian, D., Chitty-Lopez, M. & Leiding, J. W. Precision therapy for the treatment of primary immunodysregulatory diseases. Immunol. Allergy Clin. North. Am. 40, 511–526 (2020).
Leiding, J. W. et al. Monogenic early-onset lymphoproliferation and autoimmunity: natural history of STAT3 gain-of-function syndrome. J. Allergy Clin. Immunol. 151, 1081–1095 (2023).
Chan, A. Y. et al. Hematopoietic cell transplantation in patients with primary immune regulatory disorders (PIRD): a primary immune deficiency treatment consortium (PIDTC) survey. Front. Immunol. 11, 239 (2020).
Tao, Z. et al. Impact of T cell characteristics on CAR-T cell therapy in hematological malignancies. Blood Cancer J. 14, 213 (2024).
Baguet, C., Larghero, J. & Mebarki, M. Early predictive factors of failure in autologous CAR T-cell manufacturing and/or efficacy in hematologic malignancies. Blood Adv. 8, 337–342 (2024).
Arcangeli, S. et al. CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. J. Clin. Invest. 132, e150807 (2022).
Robinson, G. A. et al. Disease-associated and patient-specific immune cell signatures in juvenile-onset systemic lupus erythematosus: patient stratification using a machine-learning approach. Lancet Rheumatol. 2, e485–e496 (2020).
Wat, J. & Barmettler, S. Hypogammaglobulinemia after chimeric antigen receptor (CAR) T-cell therapy: characteristics, management, and future directions. J. Allergy Clin. Immunol. Pract. 10, 460–466 (2022).
Angelidakis, G. et al. Humoral immunity and antibody responses against diphtheria, tetanus, and pneumococcus after immune effector cell therapies: a prospective study. Vaccines 12, 1070 (2024).
Abdel Rahman, Z. et al. Impact of anti-CD19 CAR-T axicabtagene ciloleucel on vaccine titers of DTaP and MMR. Blood 134, 5610–5610 (2019).
Bansal, R. et al. Vaccine titers in lymphoma patients receiving chimeric antigen receptor T cell therapy. Blood 138, 3857–3857 (2021).
Reynolds, G., Hall, V. G. & Teh, B. W. Vaccine schedule recommendations and updates for patients with hematologic malignancy post-hematopoietic cell transplant or CAR T-cell therapy. Transpl. Infect. Dis. 25, e14109 (2023).
Walti, C. S. et al. Antibodies against vaccine-preventable infections after CAR-T cell therapy for B cell malignancies. JCI Insight 6, e146743 (2021).
Bass, A. R. et al. 2022 American College of Rheumatology guideline for vaccinations in patients with rheumatic and musculoskeletal diseases. Arthritis Care Res. 75, 449–464 (2023).
Hill, J. A. & Seo, S. K. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood 136, 925–935 (2020).
Los-Arcos, I. et al. Recommendations for screening, monitoring, prevention, and prophylaxis of infections in adult and pediatric patients receiving CAR T-cell therapy: a position paper. Infection 49, 215–231 (2021).
Hayden, P. J. et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the joint accreditation committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann. Oncol. 33, 259–275 (2022).
Khawaja, F. et al. ASH-ASTCT COVID-19 vaccination for HCT and CAR T cell recipients: frequently asked questions. hematology.org https://www.hematology.org/covid-19/ash-astct-covid-19-vaccination-for-hct-and-car-t-cell-recipients (2022).
Hill, J. A. et al. SARS-CoV-2 vaccination in the first year after hematopoietic cell transplant or chimeric antigen receptor T-cell therapy: a prospective, multicenter, observational study. Clin. Infect. Dis. 79, 542–554 (2024).
Kinoshita, H. et al. T cell immune response to influenza vaccination when administered prior to and following autologous chimeric antigen receptor-modified T cell therapy. Transpl. Cell Ther. 31, 327–338 (2025).
Walti, C. S. et al. Humoral immunogenicity of the seasonal influenza vaccine before and after CAR-T-cell therapy: a prospective observational study. J. Immunother. Cancer 9, e003428 (2021).
Summary of risk-based pneumococcal vaccination recommendations. cdc.gov https://www.cdc.gov/pneumococcal/hcp/vaccine-recommendations/risk-indications.html (2025).
Abid, M. A. & Abid, M. B. SARS-CoV-2 vaccine response in CAR T-cell therapy recipients: a systematic review and preliminary observations. Hematol. Oncol. 40, 287–291 (2022).
Gossi, S. et al. Humoral responses to repetitive doses of COVID-19 mRNA vaccines in patients with CAR-T-cell therapy. Cancers 14, 3527 (2022).
Meir, J., Abid, M. A. & Abid, M. B. State of the CAR-T: risk of infections with chimeric antigen receptor T-cell therapy and determinants of SARS-CoV-2 vaccine responses. Transpl. Cell Ther. 27, 973–987 (2021).
Lee, D. et al. Pneumococcal conjugate vaccine does not induce humoral response when administrated within the six months after CD19 CAR T-cell therapy. Transpl. Cell Ther. 29, 277.e1–277.e9 (2023).
Baden, L. R. et al. Prevention and treatment of cancer-related infections, version 3.2024, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 22, 617–644 (2024).
Fajgenbaum, D. C. & June, C. H. Cytokine storm. N. Engl. J. Med. 383, 2255–2273 (2020).
Bonifant, C. L., Jackson, H. J., Brentjens, R. J. & Curran, K. J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 3, 16011 (2016).
Brudno, J. N. & Kochenderfer, J. N. Current understanding and management of CAR T cell-associated toxicities. Nat. Rev. Clin. Oncol. 21, 501–521 (2024).
Morris, E. C., Neelapu, S. S., Giavridis, T. & Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 22, 85–96 (2022).
Hines, M. R. et al. Immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome. Transpl. Cell Ther. 29, 438.e1–438.e16 (2023).
Teachey, D. T. et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 6, 664–679 (2016).
Gardner, R. A. et al. Preemptive mitigation of CD19 CAR T-cell cytokine release syndrome without attenuation of antileukemic efficacy. Blood 134, 2149–2158 (2019).
Kadauke, S. et al. Risk-adapted preemptive tocilizumab to prevent severe cytokine release syndrome after CTL019 for pediatric B-cell acute lymphoblastic leukemia: a prospective clinical trial. J. Clin. Oncol. 39, 920–930 (2021).
Duncan, B. B., Dunbar, C. E. & Ishii, K. Applying a clinical lens to animal models of CAR-T cell therapies. Mol. Ther. Methods Clin. Dev. 27, 17–31 (2022).
Hay, K. A. et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 130, 2295–2306 (2017).
Velasco, R., Mussetti, A., Villagran-Garcia, M. & Sureda, A. CAR T-cell-associated neurotoxicity in central nervous system hematologic disease: is it still a concern? Front. Neurol. 14, 1144414 (2023).
Bachy, E. et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat. Med. 28, 2145–2154 (2022).
Myers, R. M. et al. Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for B-ALL. J. Clin. Oncol. 40, 932–944 (2022).
Ravich, J. W. et al. Impact of high disease burden on survival in pediatric patients with B-ALL treated with tisagenlecleucel. Transpl. Cell Ther. 28, 73.e1–73.e9 (2022).
Schwingen, N. R. et al. Distinct safety and toxicity profile of CD19-directed CAR T-cell therapy in systemic lupus erythematosus versus B-cell lymphoma — a single-center experience. Blood 144, 4835–4835 (2024).
Vora, S. B. et al. Infectious complications following CD19 chimeric antigen receptor T-cell therapy for children, adolescents, and young adults. Open. Forum Infect. Dis. 7, ofaa121 (2020).
Livingston, B., Bonner, A. & Pope, J. Differences in clinical manifestations between childhood-onset lupus and adult-onset lupus: a meta-analysis. Lupus 20, 1345–1355 (2011).
Perna, F. et al. CAR T-cell toxicities: from bedside to bench, how novel toxicities inform laboratory investigations. Blood Adv. 8, 4348–4358 (2024).
Hagen, M. et al. Local immune effector cell-associated toxicity syndrome in CAR T-cell treated patients with autoimmune disease: an observational study. Lancet Rheumatol. 7, e424–e433 (2025).
Murugesan, V. et al. Comparison of histomorphological indices between adult and pediatric patients in response to induction therapy. Cureus 16, e66673 (2024).
Verdun, N. & Marks, P. Secondary cancers after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 390, 584–586 (2024).
Elsallab, M. et al. Second primary malignancies after commercial CAR T-cell therapy: analysis of the FDA adverse events reporting system. Blood 143, 2099–2105 (2024).
Lamble, A. J. et al. Risk of T-cell malignancy after CAR T-cell therapy in children, adolescents, and young adults. Blood Adv. 8, 3544–3548 (2024).
Jadlowsky, J. K. et al. Long-term safety of lentiviral or gammaretroviral gene-modified T cell therapies. Nat. Med 31, 1134–1144 (2025).
Edens, C. The impact of pediatric rheumatic diseases on sexual health, family planning, and pregnancy. Rheum. Dis. Clin. North. Am. 48, 113–140 (2022).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Anderson, N. D. et al. Transcriptional signatures associated with persisting CD19 CAR-T cells in children with leukemia. Nat. Med. 29, 1700–1709 (2023).
Ligon, J. A. et al. Fertility and CAR T-cells: current practice and future directions. Transpl. Cell Ther. 28, 605 e1–605 e8 (2022).
Oktay, K. et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 36, 1994–2001 (2018).
Nahata, L., Sivaraman, V. & Quinn, G. P. Fertility counseling and preservation practices in youth with lupus and vasculitis undergoing gonadotoxic therapy. Fertil. Steril. 106, 1470–1474 (2016).
Leavitt, M., Adeleye, A. & Edens, C. Preserving fertility in people with rheumatic diseases. J. Clin. Rheumatol. 30, S13–S24 (2024).
O’Leary, M. C. et al. FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin. Cancer Res. 25, 1142–1146 (2019).
Ringold, S., Consolaro, A. & Ardoin, S. P. Outcome measures in pediatric rheumatic disease. Rheum. Dis. Clin. North Am. 47, 655–668 (2021).
Kim, H. et al. Performance of the 2016 ACR-EULAR myositis response criteria in juvenile dermatomyositis therapeutic trials and consensus profiles. Rheumatology 62, 3680–3689 (2023).
Foeldvari, I. et al. Proposed response parameters for twelve-month drug trial in juvenile systemic sclerosis: results of the Hamburg international consensus meetings.Arthritis Care Res. 75, 2453–2462 (2023).
Brunner, H. I. et al. American College of Rheumatology provisional criteria for clinically relevant improvement in children and adolescents with childhood-onset systemic lupus erythematosus. Arthritis Care Res. 71, 579–590 (2019).
Smith, E. M. D. et al. Defining remission in childhood-onset lupus: PReS-endorsed consensus definitions by an international task force. Clin. Immunol. 263, 110214 (2024).
Lazarevic, D. et al. The PRINTO criteria for clinically inactive disease in juvenile dermatomyositis. Ann. Rheum. Dis. 72, 686–693 (2013).
Del Gaizo, V. & Kohlheim, M. Patient engagement in pediatric rheumatology research. Rheum. Dis. Clin. North Am. 48, 1–13 (2022).
Weitzman, E. R. et al. Adding patient-reported outcomes to a multisite registry to quantify quality of life and experiences of disease and treatment for youth with juvenile idiopathic arthritis. J. Patient Rep. Outcomes 2, 1 (2018).
Gonzalez Sepulveda, J. M. et al. Preferences for potential benefits and risks for gene therapy in the treatment of sickle cell disease. Blood Adv. 7, 7371–7381 (2023).
Zhao, Z. et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28, 415–428 (2015).
Rotte, A. et al. Dose-response correlation for CAR-T cells: a systematic review of clinical studies. J. Immunother. Cancer 10, e005678 (2022).
Stefanski, H. E. et al. Higher doses of tisagenlecleucel are associated with improved outcomes: a report from the pediatric real-world CAR consortium. Blood Adv. 7, 541–548 (2023).
Gauthier, J. et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood 137, 323–335 (2021).
Holland, E. M. et al. Efficacy of second CAR-T (CART2) infusion limited by poor CART expansion and antigen modulation. J. Immunother. Cancer 10, e004483 (2022).
Turtle, C. J. et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 126, 2123–2138 (2016).
Hill, J. A. et al. Durable preservation of antiviral antibodies after CD19-directed chimeric antigen receptor T-cell immunotherapy. Blood Adv. 3, 3590–3601 (2019).
Bodansky, A. et al. Unveiling the proteome-wide autoreactome enables enhanced evaluation of emerging CAR T cell therapies in autoimmunity. J. Clin. Invest. 134, e180012 (2024).
Hill, J. A. et al. Anti-HLA antibodies in recipients of CD19 versus BCMA-targeted CAR T-cell therapy. Am. J. Transpl. 23, 416–422 (2023).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl. 25, 625–638 (2019).
Rejeski, K. et al. Immune effector cell-associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood 142, 865–877 (2023).
Costenbader, K. H. et al. Development and initial validation of a self-assessed lupus organ damage instrument. Arthritis Care Res. 62, 559–568 (2010).
Rider, L. G. et al. Damage extent and predictors in adult and juvenile dermatomyositis and polymyositis as determined with the myositis damage index. Arthritis Rheum. 60, 3425–3435 (2009).
Reeve, B. B. et al. Validity and reliability of the pediatric patient-reported outcomes version of the common terminology criteria for adverse events. J. Natl Cancer Inst. 112, 1143–1152 (2020).
McNerney, K. O. et al. INSPIRED symposium part 3: prevention and management of pediatric chimeric antigen receptor T cell-associated emergent toxicities. Transpl. Cell Ther. 30, 38–55 (2024).
Pennisi, M. et al. Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells. Blood Adv. 5, 3397–3406 (2021).
Hashmi, H. et al. Venous thromboembolism associated with CD19-directed CAR T-cell therapy in large B-cell lymphoma. Blood Adv. 4, 4086–4090 (2020).
Kammeyer, R. et al. Cognitive dysfunction in pediatric systemic lupus erythematosus: current knowledge and future directions. Child. Neuropsychol. 30, 818–846 (2024).
Samuels, S. et al. Pediatric efficacy extrapolation in drug development submitted to the US Food and Drug Administration 2015–2020. J. Clin. Pharmacol. 63, 307–313 (2023).
Food and Drug Administration. Pediatric extrapolation guidance for industry https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e11a-pediatric-extrapolation (2024).
Food and Drug Administration. Risk evaluation and mitigation strategies (REMS) for autologous chimeric antigen receptor (CAR) T cell immunotherapies modified to minimize burden on healthcare delivery system https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/risk-evaluation-and-mitigation-strategies-rems-autologous-chimeric-antigen-receptor-car-t-cell (2024).
Author information
Authors and Affiliations
Consortia
Contributions
S.P., K.A., H.K., S.W.J., J.C.C., S.P.A., H.W., C.L.B, N.N.S. and M.L. researched data for the article and wrote the manuscript. All authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H.W. has stock ownership in Regatta Bio and has pending patent applications related to cellular therapies. C.L.B. has awarded and pending patent applications describing the use of engineered cells as therapeutics and has received research support from Merck, Sharp and Dohme, Bristol-Myers Squibb and Kiadis Pharma. J.C.C. is a consultant at Sana Biotechnology and Synthekine and is a Paediatric SLE Advisory Board Member for Bristol-Myers Squibb. H.K. is supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Arthritis and Musculoskeletal and Skin diseases (NIAMS) (AR041215), is a juvenile myositis expert panel member for Cabaletta Bio and is part of NIAMS CRADA with provision of a drug (deucravacitinib) with Bristol-Myers Squibb, and previously part of NIAMS CRADA, with study support and the drug (baricitinib) with Eli Lilly and Company. L.L is supported by ZIA AR04121404. M.K. receives author royalties from Wolter-Kluwer (UpToDate) and is a consultant for Chiesi Pharmaceuticals and M3 Global Research. R.A.C. is supported by ZIAAR041184. C.E. is a site PI of the Cabaletta Bio-sponsored RESET-SLE CAR T cell trial for SLE. S.W.J. is a consultant for Merck, IgM BioSciences and Sail BioMedicines and previously served as a consultant for Bristol-Myers Squib, Variant Bio and ChemoCentryx. S.W.J. has funding provided by the National Institutes of Health (1R01DK136980 and 1K24AR085177) and Lupus Research Alliance (Lupus Mechanisms and Targets Award and Global Team Science Award). S.P. receives support for the conducting of clinical trials through Boston Children’s Hospital from Atara and Jasper, is the inventor of IP related to development of third-party viral specific T cells programme with all rights assigned to Memorial Sloan Kettering Cancer Center, has received honoraria from Pierre Fabre, has engaged in consulting with Atara Biotherapeutics, Ensomo, HEOR, Pierre Fabre and VOR, is a DSMB member for Stanford University and NYBC and has equity interest in Regatta Biotherapies. N.N.S. receives research funding from Lentigen, VOR Bio and CARGO Therapeutics, has attended advisory board meetings (no honoraria) for VOR, ImmunoACT, and Sobi, receives royalties from CARGO and funding that supports this work was provided in part by the Intramural Research Program, Center of Cancer Research, National Cancer Institute and NIH Clinical Center, National Institutes of Health (ZIA BC 011823, N.N.S). K.A. has served on a scientific advisory board of Cabaletta Bio, completed paid consulting for Cabaletta Bio, serves as site PI of the Cabaletta Bio sponsored RESET-Myositis trial, serves as the Vice Chair of the Juvenile Dermatomyositis Committee for the Childhood Arthritis & Rheumatology Research Alliance (paid consultant position), has received honoraria/travel reimbursement from the Rheumatology Research Foundation and the American College of Rheumatology and receives research funding from the Rheumatology Research Foundation, Childhood Arthritis & Rheumatology Research Alliance/Arthritis Foundation, Chan Zuckerberg Initiative, Cure JM Foundation, Patient-Centered Outcomes Research Institute, and National Institutes of Health/Technical Resources International. M.L. participates in sponsored research funded by Luminary Biotherapeutics. The Integrated Multidisciplinary Paediatric Autoimmunity and Cell Therapy (IMPACT) Working Group has no competing interests to disclose. The remaining authors do not have any competing interests to disclose. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services or the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Terry Fry and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wobma, H., Ardoin, S.P., Bonifant, C.L. et al. CAR T cell therapy for children with rheumatic disease: the time is now. Nat Rev Rheumatol 21, 494–506 (2025). https://doi.org/10.1038/s41584-025-01272-3
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41584-025-01272-3