Abstract
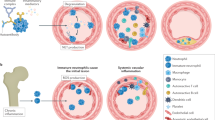
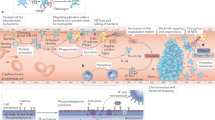
Platelets are central players in inflammatory and thrombotic responses that drive the onset and progression of rheumatic diseases. In particular, they regulate immunothrombosis, a defence mechanism in which the immune and blood-clotting systems cooperate to contain infections or vascular damage. Although immunothrombosis can help to preserve blood-vessel integrity and promote healing, it becomes harmful when exaggerated or chronic. In rheumatic diseases, such as systemic lupus erythematosus, systemic sclerosis and antiphospholipid syndrome, immunothrombosis contributes to persistent inflammation, abnormal blood-clot formation and long-term damage to the small blood vessels. It has also been implicated in maintaining autoimmune responses to autoantigens released by neutrophils. Platelets are among the first responders to vascular injury and influence the activity of immune cells, particularly neutrophils, by promoting the formation of neutrophil extracellular traps. Platelets express proteins such as P-selectin and the damage-associated molecule high-mobility group box 1 (HMGB1), which have distinct and non-redundant roles, both via direct interactions locally at sites of vascular damage and systemically via the release of extracellular vesicles. Understanding how platelets contribute to vascular inflammation and clotting in autoimmune settings elucidates disease mechanisms and might lead to the identification of new therapeutic targets.
Key points
-
Immunothrombosis integrates innate immune defence with coagulation, when dysregulated, it sustains maladaptive immune responses, driving inflammation, thrombotic complications and pathological tissue remodelling — highlighting its relevance as a key therapeutic target.
-
Platelets are central orchestrators of immunothrombosis, bridging vascular injury and immune activation in rheumatic diseases.
-
Although the hallmarks of immunothrombosis are shared across rheumatic diseases, the cellular mediators and initiating pathways vary according to disease-specific inflammatory contexts.
-
Pharmacological targeting of immunothrombosis holds promise not only for reducing autoimmune-driven cardiovascular risk but also for controlling chronic inflammation and limiting tissue damage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Stark, K. & Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 18, 666–682 (2021).
Olumuyiwa-Akeredolu, O. O., Page, M. J., Soma, P. & Pretorius, E. Platelets: emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat. Rev. Rheumatol. 15, 237–248 (2019).
Scherlinger, M., Richez, C., Tsokos, G. C., Boilard, E. & Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 23, 495–510 (2023).
Nicolai, L., Pekayvaz, K. & Massberg, S. Platelets: orchestrators of immunity in host defense and beyond. Immunity 57, 957–972 (2024).
Boilard, E. & Bellio, M. Platelet extracellular vesicles and the secretory interactome join forces in health and disease. Immunol. Rev. 312, 38–51 (2022).
Boulaftali, Y., Massberg, S. & Nicolai, L. Platelets in vascular inflammation: fire-fighters or pyromaniacs? Curr Opin Hematol 32, 221–230.(2025).
Maugeri, N. et al. Platelet microparticles sustain autophagy-associated activation of neutrophils in systemic sclerosis. Sci. Transl. Med. 10, eaao3089 (2018).
Manfredi, A. A. et al. Platelet phagocytosis via P-selectin glycoprotein ligand 1 and accumulation of microparticles in systemic sclerosis. Arthritis Rheumatol. 74, 318–328 (2022).
Linge, P., Fortin, P. R., Lood, C., Bengtsson, A. A. & Boilard, E. The non-haemostatic role of platelets in systemic lupus erythematosus. Nat. Rev. Rheumatol. 14, 195–213 (2018).
Melki, I. et al. Platelets release mitochondrial antigens in systemic lupus erythematosus. Sci. Transl. Med. 13, eaav5928 (2021).
Raschi, E., Borghi, M. O., Tedesco, F. & Meroni, P. L. Antiphospholipid syndrome pathogenesis in 2023: an update of new mechanisms or just a reconsideration of the old ones? Rheumatology 63, SI4–SI13 (2024).
Tektonidou, M. G. et al. Kidney whole-transcriptome profiling in primary antiphospholipid syndrome reveals complement, interferons and NETs-related gene expression. Rheumatology 63, 3184–3190 (2024).
Machlus, K. R. & Italiano, J. E. Jr The incredible journey: from megakaryocyte development to platelet formation. J. Cell Biol. 201, 785–796 (2013).
Poscablo, D. M. et al. An age-progressive platelet differentiation path from hematopoietic stem cells causes exacerbated thrombosis. Cell 187, 3090–3107.e21 (2024).
Heazlewood, S. Y. et al. High ploidy large cytoplasmic megakaryocytes are hematopoietic stem cells regulators and essential for platelet production. Nat. Commun. 14, 2099 (2023).
Anjum, A. et al. Aging platelets shift their hemostatic properties to inflammatory functions. Blood 145, 1568–1582 (2025).
Zhao, X. et al. Highly efficient platelet generation in lung vasculature reproduced by microfluidics. Nat. Commun. 14, 4026 (2023).
Lefrancais, E. et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109 (2017).
Puhm, F., Laroche, A. & Boilard, E. Diversity of megakaryocytes. Arterioscl. Thromb. Vasc. Biol. 43, 2088–2098 (2023).
Conrad, C. et al. Decoding functional hematopoietic progenitor cells in the adult human lung. Blood 145, 1975–1986 (2025).
Livada, A. C. et al. Long-lived lung megakaryocytes contribute to platelet recovery in thrombocytopenia models. J. Clin. Invest. 134, e181111 (2024).
Rossaint, J. et al. Platelets orchestrate the resolution of pulmonary inflammation in mice by T reg cell repositioning and macrophage education. J. Exp. Med. 218, e20201353 (2021).
Pariser, D. N. et al. Lung megakaryocytes are immune modulatory cells. J. Clin. Invest. 131, e137377 (2021).
Qiu, J. et al. Lung megakaryocytes engulf inhaled airborne particles to promote intrapulmonary inflammation and extrapulmonary distribution. Nat. Commun. 15, 7396 (2024).
Valet, C. et al. Sepsis promotes splenic production of a protective platelet pool with high CD40 ligand expression. J. Clin. Invest. 132, e153920 (2022).
Maher, T. M. Interstitial lung disease: a review. JAMA 331, 1655–1665 (2024).
Joy, G. M. et al. Prevalence, imaging patterns and risk factors of interstitial lung disease in connective tissue disease: a systematic review and meta-analysis. Eur. Respir. Rev. 32, 220210 (2023).
Zhou, Y. et al. Megakaryocytes participate in the occurrence of bleomycin-induced pulmonary fibrosis. Cell Death Dis. 10, 648 (2019).
Nicolai, L. et al. Vascular surveillance by haptotactic blood platelets in inflammation and infection. Nat. Commun. 11, 5778 (2020).
Italiano, J. E. Jr et al. Mechanisms and implications of platelet discoid shape. Blood 101, 4789–4796 (2003).
Patel-Hett, S. et al. The spectrin-based membrane skeleton stabilizes mouse megakaryocyte membrane systems and is essential for proplatelet and platelet formation. Blood 118, 1641–1652 (2011).
Gupta, S. et al. Hemostasis vs. homeostasis: platelets are essential for preserving vascular barrier function in the absence of injury or inflammation. Proc. Natl Acad. Sci. USA 117, 24316–24325 (2020).
Ho-Tin-Noe, B., Le Chapelain, O. & Camerer, E. Platelets maintain vascular barrier function in the absence of injury or inflammation. J. Thromb. Haemost. 19, 1145–1148 (2021).
Koszalka, P. et al. Targeted disruption of cd73/ecto-5’-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ. Res. 95, 814–821 (2004).
Chaurasia, S. N., Kushwaha, G., Pandey, A. & Dash, D. Human platelets express functional ectonucleotidases that restrict platelet activation signaling. Biochem. Biophys. Res. Commun. 527, 104–109 (2020).
Thienel, M. et al. Immobility-associated thromboprotection is conserved across mammalian species from bear to human. Science 380, 178–187 (2023).
Vachon, L. et al. Platelet extracellular vesicles preserve lymphatic endothelial cell integrity and enhance lymphatic vessel function. Commun. Biol. 7, 975 (2024).
Sixma, J. J. & de Groot, P. G. Regulation of platelet adhesion to the vessel wall. Ann. N. Y. Acad. Sci. 714, 190–199 (1994).
Monroe, D. M., Hoffman, M. & Roberts, H. R. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 22, 1381–1389 (2002).
Risman, R. A., Sen, M., Tutwiler, V. & Hudson, N. E. Deconstructing fibrin(ogen) structure. J. Thromb. Haemost. 23, 368–380 (2025).
Aggarwal, A., Jennings, C. L., Manning, E. & Cameron, S. J. Platelets at the vessel wall in non-thrombotic disease. Circ. Res. 132, 775–790 (2023).
Oshinowo, O., Azer, S. S., Lin, J. & Lam, W. A. Why platelet mechanotransduction matters for hemostasis and thrombosis. J. Thromb. Haemost. 21, 2339–2353 (2023).
Heijnen, H. & van der Sluijs, P. Platelet secretory behaviour: as diverse as the granules… or not? J. Thromb. Haemost. 13, 2141–2151 (2015).
Semple, J. W., Italiano, J. E. Jr & Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 11, 264–274 (2011).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Engelmann, B. & Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 13, 34–45 (2013).
Sreeramkumar, V. et al. Neutrophils scan for activated platelets to initiate inflammation. Science 346, 1234–1238 (2014).
Kaiser, R., Escaig, R. & Nicolai, L. Hemostasis without clot formation: how platelets guard the vasculature in inflammation, infection, and malignancy. Blood 142, 1413–1425 (2023).
Marchesi, V. T. Some electron microscopic observations on interactions between leukocytes, platelets, and endothelial cells in acute inflammation. Ann. N. Y. Acad. Sci. 116, 774–788 (1964).
Gaertner, F. et al. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell 171, 1368–1382.e23 (2017).
Ho-Tin-Noe, B., Boulaftali, Y. & Camerer, E. Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood 131, 277–288 (2018).
Kaiser, R. et al. Mechanosensing via a GpIIb/Src/14-3-3ζ axis critically regulates platelet migration in vascular inflammation. Blood 141, 2973–2992 (2023).
Deppermann, C. et al. Platelet secretion is crucial to prevent bleeding in the ischemic brain but not in the inflamed skin or lung in mice. Blood 129, 1702–1706 (2017).
Piccardoni, P. et al. Platelet/polymorphonuclear leukocyte adhesion: a new role for SRC kinases in Mac-1 adhesive function triggered by P-selectin. Blood 98, 108–116 (2001).
Hidalgo, A. et al. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat. Med. 15, 384–391 (2009).
Zarbock, A., Ley, K., McEver, R. P. & Hidalgo, A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118, 6743–6751 (2011).
Lam, F. W., Burns, A. R., Smith, C. W. & Rumbaut, R. E. Platelets enhance neutrophil transendothelial migration via P-selectin glycoprotein ligand-1. Am. J. Physiol. Heart Circ. Physiol. 300, H468–H475 (2011).
Dib, P. R. B. et al. Innate immune receptors in platelets and platelet-leukocyte interactions. J. Leukoc. Biol. 108, 1157–1182 (2020).
Manfredi, A. A. et al. Anti-TNFɑ agents curb platelet activation in patients with rheumatoid arthritis. Ann. Rheum. Dis. 75, 1511–1520 (2016).
van Loo, G. & Bertrand, M. J. M. Death by TNF: a road to inflammation. Nat. Rev. Immunol. 23, 289–303 (2023).
Hu, S. et al. The biological disease-modifying antirheumatic drugs and the risk of cardiovascular events: a systematic review and meta-analysis. Mediators Inflamm. 2021, 7712587 (2021).
Liew, J. W. et al. The association of TNF inhibitor use with incident cardiovascular events in radiographic axial spondyloarthritis. Semin. Arthritis Rheum. 68, 152482 (2024).
Stark, K. et al. Antibodies and complement are key drivers of thrombosis. Immunity 57, 2140–2156.e10 (2024).
Maugeri, N., Rovere-Querini, P. & Manfredi, A. A. Disruption of a regulatory network consisting of neutrophils and platelets fosters persisting inflammation in rheumatic diseases. Front. Immunol. 7, 182 (2016).
Itkin, T. et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328 (2016).
Petzold, T. et al. Neutrophil “plucking” on megakaryocytes drives platelet production and boosts cardiovascular disease. Immunity 55, 2285–2299.e7 (2022).
Cunin, P. et al. Megakaryocyte emperipolesis mediates membrane transfer from intracytoplasmic neutrophils to platelets. eLife 8, e44031 (2019).
Centurione, L. et al. Increased and pathologic emperipolesis of neutrophils within megakaryocytes associated with marrow fibrosis in GATA-1low mice. Blood 104, 3573–3580 (2004).
Sims, M. C. et al. Novel manifestations of immune dysregulation and granule defects in gray platelet syndrome. Blood 136, 1956–1967 (2020).
Khatib-Massalha, E. et al. Defective neutrophil clearance in JAK2V617F myeloproliferative neoplasms drives myelofibrosis via immune checkpoint CD24. Blood https://doi.org/10.1182/blood.2024027455 (2025).
De Giovanni, M. et al. GPR35 promotes neutrophil recruitment in response to serotonin metabolite 5-HIAA. Cell 185, 815–830.e19 (2022).
Maugeri, N. et al. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J. Thromb. Haemost. 4, 1323–1330 (2006).
Maugeri, N., de Gaetano, G., Barbanti, M., Donati, M. B. & Cerletti, C. Prevention of platelet-polymorphonuclear leukocyte interactions: new clues to the antithrombotic properties of parnaparin, a low molecular weight heparin. Haematologica 90, 833–839 (2005).
Maugeri, N. & Manfredi, A. A. Tissue factor expressed by neutrophils: another piece in the vascular inflammation puzzle. Semin. Thromb. Hemost. 41, 728–736 (2015).
Rinder, H. M., Bonan, J. L., Rinder, C. S., Ault, K. A. & Smith, B. R. Dynamics of leukocyte-platelet adhesion in whole blood. Blood 78, 1730–1737 (1991).
Ramirez, G. A., Manfredi, A. A. & Maugeri, N. Misunderstandings between platelets and neutrophils build in chronic inflammation. Front. Immunol. 10, 2491 (2019).
Joseph, J. E., Harrison, P., Mackie, I. J., Isenberg, D. A. & Machin, S. J. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br. J. Haematol. 115, 451–459 (2001).
NaveenKumar, S. K. et al. Low ectonucleotidase activity and increased neutrophil-platelet aggregates in patients with antiphospholipid syndrome. Blood 143, 1193–1197 (2024).
Ueno, K. et al. Circulating platelet-neutrophil aggregates play a significant role in Kawasaki disease. Circ. J. 79, 1349–1356 (2015).
Michelson, A. D., Barnard, M. R., Krueger, L. A., Valeri, C. R. & Furman, M. I. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 104, 1533–1537 (2001).
Liu, Z. L., Bresette, C., Aidun, C. K. & Ku, D. N. SIPA in 10 milliseconds: VWF tentacles agglomerate and capture platelets under high shear. Blood Adv. 6, 2453–2465 (2022).
Banka, A. L. et al. Cargo-free particles divert neutrophil-platelet aggregates to reduce thromboinflammation. Nat. Commun. 14, 2462 (2023).
Maugeri, N. et al. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and β2 integrin-dependent cell clearance program. Blood 113, 5254–5265 (2009).
Maugeri, N. et al. Clearance of circulating activated platelets in polycythemia vera and essential thrombocythemia. Blood 118, 3359–3366 (2011).
Senchenkova, E. Y. et al. Novel role for the AnxA1-Fpr2/ALX signaling axis as a key regulator of platelet function to promote resolution of inflammation. Circulation 140, 319–335 (2019).
Manfredi, A. A., Covino, C., Rovere-Querini, P. & Maugeri, N. Instructive influences of phagocytic clearance of dying cells on neutrophil extracellular trap generation. Clin. Exp. Immunol. 179, 24–29 (2015).
Manfredi, A. A., Ramirez, G. A., Rovere-Querini, P. & Maugeri, N. The neutrophil’s choice: phagocytose vs make neutrophil extracellular traps. Front. Immunol. 9, 288 (2018).
McDonald, B., Urrutia, R., Yipp, B. G., Jenne, C. N. & Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 12, 324–333 (2012).
Keulen, G. M., Huckriede, J., Wichapong, K. & Nicolaes, G. A. F. Histone activities in the extracellular environment: regulation and prothrombotic implications. Curr. Opin. Hematol. 31, 230–237 (2024).
McDonald, B. et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129, 1357–1367 (2017).
Ngo, A. T. et al. Platelet factor 4 limits neutrophil extracellular trap- and cell-free DNA-induced thrombogenicity and endothelial injury. JCI Insight 8, e171054 (2023).
Kaiser, R., Gold, C. & Stark, K. Recent advances in immunothrombosis and thromboinflammation. Thromb Haemost https://doi.org/10.1055/a-2523-1821 (2025).
Maugeri, N. et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 12, 2074–2088 (2014).
Vogel, S. et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Invest. 125, 4638–4654 (2015).
Stark, K. et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 128, 2435–2449 (2016).
Maugeri, N. & Manfredi, A. A. Platelet HMGB1 steers intravascular immunity and thrombosis. J. Thromb. Haemost. 22, 3336–3345 (2024).
von Bruhl, M. L. et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 209, 819–835 (2012).
Tadie, J. M., Bae, H. B., Banerjee, S., Zmijewski, J. W. & Abraham, E. Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am. J. Physiol. Cell Physiol. 302, C249–C256 (2012).
Josefsson, E. C. Platelet intrinsic apoptosis. Thromb. Res. 231, 206–213 (2023).
Denorme, F. & Campbell, R. A. Procoagulant platelets: novel players in thromboinflammation. Am. J. Physiol. Cell Physiol. 323, C951–C958 (2022).
Puhm, F., Boilard, E. & Machlus, K. R. Platelet extracellular vesicles: beyond the blood. Arterioscler. Thromb. Vasc. Biol. 41, 87–96 (2021).
Hottz, E. D. et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 122, 3405–3414 (2013).
Cornelius, D. C. et al. NLRP3 inflammasome inhibition attenuates sepsis-induced platelet activation and prevents multi-organ injury in cecal-ligation puncture. PLoS One 15, e0234039 (2020).
Marongiu, F., Ruberto, M. F., Marongiu, S., Matucci Cerinic, M. & Barcellona, D. A journey to vasculopathy in systemic sclerosis: focus on haemostasis and thrombosis. Clin. Exp. Med. 23, 4057–4064 (2023).
Beretta, L. et al. Genome-wide whole blood transcriptome profiling in a large European cohort of systemic sclerosis patients. Ann. Rheum. Dis. 79, 1218–1226 (2020).
Chen, S. et al. Integrative transcriptomic analysis of peripheral blood monocytes in systemic sclerosis and shared pathogenic pathways in autoimmune diseases. Arch. Med. Res. 56, 103072 (2025).
Gonzalez-Tajuelo, R. et al. Spontaneous pulmonary hypertension associated with systemic sclerosis in P-selectin glycoprotein ligand 1-deficient mice. Arthritis Rheumatol. 72, 477–487 (2020).
Gonzalez-Sanchez, E. et al. Targeted nanotherapy with everolimus reduces inflammation and fibrosis in scleroderma-related interstitial lung disease developed by PSGL-1 deficient mice. Br. J. Pharmacol. 179, 4534–4548 (2022).
Maugeri, N. et al. Circulating platelets as a source of the damage-associated molecular pattern HMGB1 in patients with systemic sclerosis. Autoimmunity 45, 584–587 (2012).
Maugeri, N. et al. Oxidative stress elicits platelet/leukocyte inflammatory interactions via HMGB1: a candidate for microvessel injury in sytemic sclerosis. Antioxid. Redox Signal. 20, 1060–1074 (2014).
Maugeri, N. et al. Unconventional CD147-dependent platelet activation elicited by SARS-CoV-2 in COVID-19. J. Thromb. Haemost. 20, 434–448 (2022).
Mouawad, J. E. et al. Role of extracellular vesicles in the propagation of lung fibrosis in systemic sclerosis. Arthritis Rheumatol. 75, 2228–2239 (2023).
Bello, N., Meyers, K. J., Workman, J., Hartley, L. & McMahon, M. Cardiovascular events and risk in patients with systemic lupus erythematosus: systematic literature review and meta-analysis. Lupus 32, 325–341 (2023).
Scherlinger, M. et al. Systemic lupus erythematosus and systemic sclerosis: all roads lead to platelets. Autoimmun. Rev. 17, 625–635 (2018).
Nhek, S. et al. Activated platelets induce endothelial cell activation via an interleukin-1β pathway in systemic lupus erythematosus. Arterioscler. Thromb. Vasc. Biol. 37, 707–716 (2017).
Cornwell, M. G. et al. Modeling of clinical phenotypes in systemic lupus erythematosus based on the platelet transcriptome and FCGR2a genotype. J. Transl. Med. 21, 247 (2023).
Kaplan, M. J. Exploring the role of neutrophil extracellular traps in systemic lupus erythematosus: a clinical case study and comprehensive review. Arthritis Rheumatol. 77, 247–252 (2025).
Tay, S. H. et al. Platelet TLR7 is essential for the formation of platelet-neutrophil complexes and low-density neutrophils in lupus nephritis. Rheumatology 63, 551–562 (2024).
Munoz-Callejas, A. et al. Low P-selectin glycoprotein ligand-1 expression in neutrophils associates with disease activity and deregulated NET formation in systemic lupus erythematosus. Int. J. Mol. Sci. 24, 6144 (2023).
Scherlinger, M. et al. Selectins impair regulatory T cell function and contribute to systemic lupus erythematosus pathogenesis. Sci. Transl. Med. 13, eabi4994 (2021).
Melki, I. et al. FcγRIIA expression accelerates nephritis and increases platelet activation in systemic lupus erythematosus. Blood 136, 2933–2945 (2020).
Lood, C. et al. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood 116, 1951–1957 (2010).
San Antonio, E. et al. PSGL-1, ADAM8, and selectins as potential biomarkers in the diagnostic process of systemic lupus erythematosus and systemic sclerosis: an observational study. Front. Immunol. 15, 1403104 (2024).
Ramirez, G. A. et al. Histone-specific CD4+ T cell plasticity in active and quiescent systemic lupus erythematosus. Arthritis Rheumatol. 76, 739–750 (2024).
Duffau, P. et al. Platelet CD154 potentiates interferon-ɑ secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci. Transl. Med. 2, 47ra63 (2010).
El Bannoudi, H. et al. Platelet LGALS3BP as a mediator of myeloid inflammation in systemic lupus erythematosus. Arthritis Rheumatol. 75, 711–722 (2023).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Lande, R. et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra19 (2011).
Kumari, P., Russo, A. J., Shivcharan, S. & Rathinam, V. A. AIM2 in health and disease: inflammasome and beyond. Immunol. Rev. 297, 83–95 (2020).
Antiochos, B. et al. The DNA sensors AIM2 and IFI16 are SLE autoantigens that bind neutrophil extracellular traps. Elife 11, e72103 (2022).
Dong, M. & Fitzgerald, K. A. DNA-sensing pathways in health, autoinflammatory and autoimmune diseases. Nat. Immunol. 25, 2001–2014 (2024).
Whittall-Garcia, L. P. et al. Circulating neutrophil extracellular trap remnants as a biomarker to predict outcomes in lupus nephritis. Lupus Sci. Med. 11, e001038 (2024).
Meng, H. et al. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol. 69, 655–667 (2017).
Grossi, C. et al. Beta 2 glycoprotein I and neutrophil extracellular traps: potential bridge between innate and adaptive immunity in anti-phospholipid syndrome. Front. Immunol. 13, 1076167 (2022).
Knight, J. S. & Erkan, D. Rethinking antiphospholipid syndrome to guide future management and research. Nat. Rev. Rheumatol. 20, 377–388 (2024).
Yalavarthi, S. et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 67, 2990–3003 (2015).
Knight, J. S. & Kanthi, Y. Mechanisms of immunothrombosis and vasculopathy in antiphospholipid syndrome. Semin. Immunopathol. 44, 347–362 (2022).
Tohidi-Esfahani, I., Mittal, P., Isenberg, D., Cohen, H. & Efthymiou, M. Platelets and thrombotic antiphospholipid syndrome. J. Clin. Med. 13, 741 (2024).
Hanata, N. & Kaplan, M. J. The role of neutrophil extracellular traps in inflammatory rheumatic diseases. Curr. Opin. Rheumatol. 37, 64–71 (2025).
Shiratori-Aso, S. & Nakazawa, D. The involvement of NETs in ANCA-associated vasculitis. Front. Immunol. 14, 1261151 (2023).
Matsumoto, K., Yasuoka, H., Yoshimoto, K., Suzuki, K. & Takeuchi, T. Platelet CXCL4 mediates neutrophil extracellular traps formation in ANCA-associated vasculitis. Sci. Rep. 11, 222 (2021).
Nishibata, Y. et al. Cathepsin C inhibition reduces neutrophil serine protease activity and improves activated neutrophil-mediated disorders. Nat. Commun. 15, 6519 (2024).
Michailidou, D. et al. Mitochondrial-mediated inflammation and platelet activation in giant cell arteritis. Clin. Immunol. 255, 109746 (2023).
Michailidou, D. et al. Neutrophil extracellular trap formation in anti-neutrophil cytoplasmic antibody-associated and large-vessel vasculitis. Clin. Immunol. 249, 109274 (2023).
Ibrahim, H. E. & De Bari, C. Giant cell arteritis: update on pathogenesis and clinical implications. Curr. Opin. Rheumatol. 37, 72–79 (2025).
Palamidas, D. A. et al. Neutrophil extracellular traps in giant cell arteritis biopsies: presentation, localization and co-expression with inflammatory cytokines. Rheumatology 61, 1639–1644 (2022).
Wright, H. L., Moots, R. J. & Edwards, S. W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 10, 593–601 (2014).
Jiang, M., Wu, W., Xia, Y., Wang, X. & Liang, J. Platelet-derived extracellular vesicles promote endothelial dysfunction in sepsis by enhancing neutrophil extracellular traps. BMC Immunol. 24, 22 (2023).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010).
Cloutier, N. et al. Platelets can enhance vascular permeability. Blood 120, 1334–1343 (2012).
Tessandier, N. et al. Platelets disseminate extracellular vesicles in lymph in rheumatoid arthritis. Arterioscler. Thromb. Vasc. Biol. 40, 929–942 (2020).
Carmona-Rivera, C. et al. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight 5, e139388 (2020).
O’Neil, L. J. et al. Neutrophil extracellular trap-associated carbamylation and histones trigger osteoclast formation in rheumatoid arthritis. Ann. Rheum. Dis. 82, 630–638 (2023).
Xu, M. et al. Platelets derived citrullinated proteins and microparticles are potential autoantibodies ACPA targets in RA patients. Front. Immunol. 14, 1084283 (2023).
Cross, A. L. et al. Circulating neutrophil extracellular trap-forming neutrophils in rheumatoid arthritis exacerbation are majority dual endothelin-1/signal peptide receptor+ subtype. Clin. Exp. Immunol. 218, 163–168 (2024).
Sowa, M. A. et al. Inhibiting the P2Y12 receptor in megakaryocytes and platelets suppresses interferon-associated responses. JACC Basic. Transl. Sci. 9, 1126–1140 (2024).
Garshick, M. S., Rosenthal, P. B., Luttrell-Williams, E., Cronstein, B. N. & Berger, J. S. Ticagrelor added to methotrexate improves rheumatoid arthritis disease severity. Rheumatology 60, 5473–5475 (2021).
Manfredi, A. A., Rovere-Querini, P., D’Angelo, A. & Maugeri, N. Low molecular weight heparins prevent the induction of autophagy of activated neutrophils and the formation of neutrophil extracellular traps. Pharmacol. Res. 123, 146–156 (2017).
Zalghout, S. & Martinod, K. Therapeutic potential of DNases in immunothrombosis: promising succor or uncertain future? J. Thromb. Haemost. 23, 760–778 (2025).
Polzin, A. et al. Long-term FXa inhibition attenuates thromboinflammation after acute myocardial infarction and stroke by platelet proteome alteration. J. Thromb. Haemost. 23, 668–683 (2025).
Schneckmann, R. et al. Rivaroxaban attenuates neutrophil maturation in the bone marrow niche. Basic. Res. Cardiol. 118, 31 (2023).
Kindberg, K. M. et al. Neutrophil extracellular traps in ST-segment elevation myocardial infarction: reduced by tocilizumab and associated with infarct size. JACC Adv. 3, 101193 (2024).
Yeung, A. K., Villacorta-Martin, C., Hon, S., Rock, J. R. & Murphy, G. J. Lung megakaryocytes display distinct transcriptional and phenotypic properties. Blood Adv. 4, 6204–6217 (2020).
Asquith, N. L. et al. The bone marrow is the primary site of thrombopoiesis. Blood 143, 272–278 (2024).
Middleton, E. A., Weyrich, A. S. & Zimmerman, G. A. Platelets in pulmonary immune responses and inflammatory lung diseases. Physiol. Rev. 96, 1211–1259 (2016).
Stone, A. P., Nikols, E., Freire, D. & Machlus, K. R. The pathobiology of platelet and megakaryocyte extracellular vesicles: A (c)lot has changed. J. Thromb. Haemost. 20, 1550–1558 (2022).
Acknowledgements
The authors acknowledge the support provided to their laboratory by the European Union — Next Generation EU — NRRP M6C2 — Investment 2.1, “Enhancement and strengthening of biomedical research in the NHS”, Project: PNRR-MR1-2022-12376638. This Review is a product of the knowledge and research environment fostered by this support.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Jason S. Knight, who co-reviewed with Somanathapura K.; Naveen Kumar, Marko Radic and Eric Boilard, for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- α-granules
-
Platelet granules that contain haemostatic proteins (such as fibrinogen and von Willebrand Factor), growth factors, angiogenic signals and adhesion molecules (such as P-selectin).
- αIIbβ3 integrin
-
Also known as GPIIb/GPIIIa. Mediates platelet aggregation by binding fibrinogen and contributing to clot formation.
- Dense granules
-
Store small molecules such as ADP, ATP, serotonin and calcium ions, which contribute to platelet aggregation and activation.
- Emperipolesis
-
A cellular process in which one living cell (such as a neutrophil) actively enters and resides within another cell (such as a megakaryocyte) without being destroyed. In the context of haematopoiesis, emperipolesis contributes to platelet heterogeneity by enabling the transfer of membrane components from neutrophils to developing platelets.
- GPIb–IX–V complex
-
A mechanoreceptor that interacts with von Willebrand Factor on the subendothelial matrix during platelet adhesion.
- High-mobility group box 1
-
(HMGB1). A damage-associated molecular pattern released from platelets that amplifies inflammation and neutrophil activation in immunothrombosis.
- Immunothrombosis
-
A physiological process in which components of the innate immune system and coagulation system, including platelets and neutrophils, cooperate to form intravascular microthrombi, which help to contain pathogens and limit their systemic spread.
- Iterative fission events
-
A stepwise process by which megakaryocytes produce platelets, involving the repeated extension and fragmentation of proplatelet projections into the bloodstream to generate mature platelets.
- ST-segment elevation myocardial infarction
-
(STEMI). A type of acute myocardial infarction characterized by persistent ST-segment elevation on electrocardiography, indicating complete or prolonged occlusion of a coronary artery.
- Thrombocytopoiesis
-
The biological process by which platelets are produced from megakaryocytes, primarily in the bone marrow but also in other tissues such as the spleen and lungs during stress or inflammation.
- Thromboinflammation
-
A pathological state that results from dysregulated or excessive immunothrombosis, in which inflammation and thrombosis amplify one another, leading to tissue damage, vascular dysfunction and organ injury.
- Thromboxane A2
-
Promotes platelet activation, aggregation and vasoconstriction.
- Von Willebrand factor
-
(vWF). Binds GPIb to mediate platelet adhesion under shear stress.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maugeri, N., Manfredi, A.A. Platelets as drivers of immunothrombosis in rheumatic diseases. Nat Rev Rheumatol 21, 478–493 (2025). https://doi.org/10.1038/s41584-025-01276-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41584-025-01276-z