Abstract
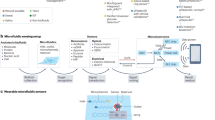
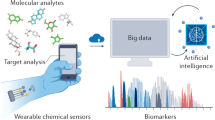
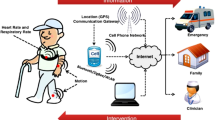
Wearable sensors are a recent paradigm in healthcare, enabling continuous, decentralized, and non- or minimally invasive monitoring of health and disease. Continuous measurements yield information-rich time series of physiological data that are holistic and clinically meaningful. Although most wearable sensors were initially restricted to biophysical measurements, the next generation of wearable devices is now emerging that enable biochemical monitoring of both small and large molecules in a variety of body fluids, such as sweat, breath, saliva, tears and interstitial fluid. Rapidly evolving data analysis and decision-making technologies through artificial intelligence has accelerated the application of wearables around the world. Although recent pilot trials have demonstrated the clinical applicability of these wearable devices, their widespread adoption will require large-scale validation across various conditions, ethical consideration and sociocultural acceptance. Successful translation of wearable devices from laboratory prototypes into clinical tools will further require a comprehensive transitional environment involving all stakeholders. The wearable device platforms must gain acceptance among different user groups, add clinical value for various medical indications, be eligible for reimbursements and contribute to public health initiatives. In this Perspective, we review state-of-the-art wearable devices for body-fluid analysis and their translation into clinical applications, and provide insight into their clinical purpose.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rizas, K. D. et al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat. Med. 28, 1823–1830 (2022).
Brasier, N. et al. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace 21, 41–47 (2019).
Kim, J., Campbell, A. S., de Ávila, B. E. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019). This paper has been one of the most successful papers providing a differentiated outlook on the use of wearable devices including their clinical application.
Ates, H. C. & Dincer, C. Wearable breath analysis. Nat. Rev. Bioeng. 1, 80–82 (2023).
Ates, H. C. et al. End-to-end design of wearable sensors. Nat. Rev. Mater. 7, 887–907 (2022). This paper provides a differentiated overview on the modularity of wearable sensors and their potential to serve various and heterogeneous needs.
Tu, J. et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-023-01059-5 (2023).
Concannon, T. W. et al. Practical guidance for involving stakeholders in health research. J. Gen. Intern. Med. 34, 458–463 (2019).
Min, J. et al. Skin-interfaced wearable sweat sensors for precision medicine. Chem. Rev. 123, 5049–5138 (2023).
Sempionatto, J. R., Lasalde-Ramírez, J. A., Mahato, K., Wang, J. & Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 6, 899–915 (2022).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 (2016).
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016).
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1, 160–171 (2018).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016). This paper demonstrated the concept of and the basic work for sweat analysis by wearable devices. It has become a highly cited and important work for the whole field.
Ghaffari, R., Aranyosi, A. J., Lee, S. P., Model, J. B. & Baker, L. B. The Gx Sweat Patch for personalized hydration management. Nat. Rev. Bioeng. 1, 5–7 (2023). This paper discusses the successful translation and commercialization of a body-fluid analysing device. It is a great example of interdisciplinary collaboration between engineers, physiologists and a business partner such as Gatorade.
Nyein, H. Y. Y. et al. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 12, 1823 (2021).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114, 4625–4630 (2017).
Wang, M. et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-022-00916-z (2022).
Ray Tyler, R. et al. Soft, skin-interfaced sweat stickers for cystic fibrosis diagnosis and management. Sci. Transl. Med. 13, eabd8109 (2021).
Ye, C. et al. A wearable aptamer nanobiosensor for non-invasive female hormone monitoring. Nat. Nanotechnol. https://doi.org/10.1038/s41565-023-01513-0 (2023).
Friedel, M. et al. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-022-00998-9 (2023).
Lipani, L. et al. Non-invasive, transdermal, path-selective and specific glucose monitoring via a graphene-based platform. Nat. Nanotechnol. 13, 504–511 (2018).
Tehrani, F. et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-022-00887-1 (2022).
Ates, H. C. et al. Biosensor-enabled multiplexed on-site therapeutic drug monitoring of antibiotics. Adv. Mater. 34, 2104555 (2022).
Maier, D. et al. Toward continuous monitoring of breath biochemistry: a paper-based wearable sensor for real-time hydrogen peroxide measurement in simulated breath. ACS Sens. 4, 2945–2951 (2019).
Nguyen, P. Q. et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 39, 1366–1374 (2021).
Jeerapan, I., Sangsudcha, W. & Phokhonwong, P. Wearable energy devices on mask-based printed electrodes for self-powered glucose biosensors. Sens. Biosensing Res. 38, 100525 (2022).
Heng, W. et al. A smart mask for exhaled breath condensate harvesting and analysis. Science 385, 954–961 (2024). This study succesfully demonstrated the application of a wearable facemask that analyses patients’ EBC.
Ge, Z. et al. Wireless and closed-loop smart dressing for exudate management and on-demand treatment of chronic wounds. Adv. Mater. 35, 2304005 (2023).
Bai, Z. et al. Smart battery-free and wireless bioelectronic platform based on a nature-skin-derived organohydrogel for chronic wound diagnosis, assessment, and accelerated healing. Nano Energy 118, 108989 (2023).
Gao, Y. et al. A flexible multiplexed immunosensor for point-of-care in situ wound monitoring. Sci. Adv. 7, eabg9614 (2021).
Pei, X. et al. A bifunctional fully integrated wearable tracker for epidermal sweat and wound exudate multiple biomarkers monitoring. Small 18, 2205061 (2022).
Jiang, Y. et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 41, 652–662 (2023).
Zhu, Y. et al. A multifunctional pro-healing zwitterionic hydrogel for simultaneous optical monitoring of pH and glucose in diabetic wound treatment. Adv. Funct. Mater. 30, 1905493 (2020).
Zheng, X. T. et al. Battery-free and AI-enabled multiplexed sensor patches for wound monitoring. Sci. Adv. 9, eadg6670 (2023).
Pang, Q. et al. Smart wound dressing for advanced wound management: real-time monitoring and on-demand treatment. Mater. Des. 229, 111917 (2023).
Ates, H. C. et al. Integrated devices for non-invasive diagnostics. Adv. Funct. Mater. 31, 2010388 (2021).
Sempionatto, J. R. et al. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 17, 1834–1842 (2017).
Kownacka, A. E. et al. Clinical evidence for use of a noninvasive biosensor for tear glucose as an alternative to painful finger-prick for diabetes management utilizing a biopolymer coating. Biomacromolecules 19, 4504–4511 (2018).
García-Carmona, L. et al. Pacifier biosensor: toward noninvasive saliva biomarker monitoring. Anal. Chem. 91, 13883–13891 (2019).
Kim, J. et al. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 74, 1061–1068 (2015).
Lim, H.-R. et al. Smart bioelectronic pacifier for real-time continuous monitoring of salivary electrolytes. Biosens. Bioelectron. 210, 114329 (2022).
Arakawa, T. et al. A wearable cellulose acetate-coated mouthguard biosensor for in vivo salivary glucose measurement. Anal. Chem. 92, 12201–12207 (2020).
Bellagambi, F. G. et al. Saliva sampling: methods and devices. An overview. Trends Anal. Chem. 124, 115781 (2020).
Zhang, J. et al. A wearable self-powered biosensor system integrated with diaper for detecting the urine glucose of diabetic patients. Sens. Actuators B 341, 130046 (2021).
Shitanda, I. et al. Self-powered diaper sensor with wireless transmitter powered by paper-based biofuel cell with urine glucose as fuel. ACS Sens. 6, 3409–3415 (2021).
Cho, J. H. et al. A smart diaper system using Bluetooth and smartphones to automatically detect urination and volume of voiding: prospective observational pilot study in an acute care hospital. J. Med. Internet Res. 23, e29979 (2021).
Li, X. et al. Smart diaper based on integrated multiplex carbon nanotube-coated electrode array sensors for in situ urine monitoring. ACS Appl. Nano Mater. 5, 4767–4778 (2022).
CIOMS Working Group XI. Patient involvement in the development, regulation and safe use of medicines (CIOMS, 2022).
Majmudar, M. D., Harrington, R. A., Brown, N. J., Graham, G. & McConnell, M. V. Clinician innovator: a novel career path in academic medicine. J. Am. Heart Assoc. 4, e001990 (2015).
FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource (Food and Drug Administration, National Institutes of Health, 2016).
Goldhahn, J., Brasier, N. & Kehoe, L. Digitalizing health trials by the Clinical Trials Transformation Initiative. Nat. Rev. Bioeng. https://doi.org/10.1038/s44222-024-00212-2 (2024).
Brasier, N. et al. Next-generation digital biomarkers: continuous molecular health monitoring using wearable devices. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2023.12.001 (2024).
Durán, C. O. et al. Implementation of digital health technology in clinical trials: the 6R framework. Nat. Med. https://doi.org/10.1038/s41591-023-02489-z (2023).
Walter, J. R., Xu, S. & Rogers, J. A. From lab to life: how wearable devices can improve health equity. Nat. Commun. 15, 123 (2024).
Jagannath, B. et al. Temporal profiling of cytokines in passively expressed sweat for detection of infection using wearable device. Bioeng. Transl. Med. 6, e10220 (2021).
Mian, Z., Hermayer, K. L. & Jenkins, A. Continuous glucose monitoring: review of an innovation in diabetes management. Am. J. Med. Sci. 358, 332–339 (2019).
Beck, R. W. et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 317, 371–378 (2017).
Brasier, N. et al. The potential of wearable sweat sensors in heart failure management. Nat. Electron. 7, 182–184 (2024).
Slavich, M. et al. Hyperhidrosis: the neglected sign in heart failure patients. Am. J. Cardiovasc. Dis. 11, 635–641 (2021).
Sempionatto, J. R. et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 5, 737–748 (2021).
Brasier, N. et al. A three-level model for therapeutic drug monitoring of antimicrobials at the site of infection. Lancet Infect. Dis. https://doi.org/10.1016/S1473-3099(23)00215-3 (2023). This concept work discusses the potential additional information that can be achieved through body-analysis through wearable devices beyond being a simple proxy for blood analysis.
Reber, E., Schönenberger, K. A., Vasiloglou, M. F. & Stanga, Z. Nutritional risk screening in cancer patients: the first step toward better clinical outcome. Front. Nutr. 8, 603936 (2021).
Niederberger, C. et al. Wearable sweat analysis to determine biological age. Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2023.02.001 (2023).
Brasier, N., Niederberger, C. & Salvatore, G. A. The sweat rate as a digital biomarker in clinical medicine beyond sports science. Soft Sci. 4, 6 (2024).
Brasier, N. et al. Towards on-skin analysis of sweat for managing disorders of substance abuse. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-024-01187-6 (2024). This comment outlines the potential of sweat analysis as a clinical body fluid by combining sweat’s biophysical and biochemical health information.
Xu, C. et al. A physicochemical-sensing electronic skin for stress response monitoring. Nat. Electron. https://doi.org/10.1038/s41928-023-01116-6 (2024). This study successfully demonstrated in a clinical study how multimodal wearable sensing using sweat and biophysical analysis can be implemented to monitor stress, thus extending actual unimodal sensors using either biophysical or biochemical analysis.
Hjelmgren, H. et al. Capillary blood sampling increases the risk of preanalytical errors in pediatric hospital care: observational clinical study. J. Spec. Pediatr. Nurs. 26, e12337 (2021).
Memon, S. F., Memon, M. & Bhatti, S. Wearable technology for infant health monitoring: a survey. IET Circuits Devices Syst. 14, 115–129 (2020).
Worth, C. et al. Continuous glucose monitoring for children with hypoglycaemia: evidence in 2023. Front. Endocrinol. 14, 1116864 (2023).
Mack, I. et al. Wearable technologies for pediatric patients with surgical infections—more than counting steps? Biosensors 12, 634 (2022).
Kruizinga, M. D. et al. Towards remote monitoring in pediatric care and clinical trials—tolerability, repeatability and reference values of candidate digital endpoints derived from physical activity, heart rate and sleep in healthy children. PLoS ONE 16, e0244877 (2021).
Rwei, A. Y. et al. A wireless, skin-interfaced biosensor for cerebral hemodynamic monitoring in pediatric care. Proc. Natl Acad. Sci. USA 117, 31674–31684 (2020).
Labrique, A. B. et al. Best practices in scaling digital health in low and middle income countries. Glob. Health 14, 103 (2018).
Chen, W. et al. Cost-effectiveness of screening for atrial fibrillation using wearable devices. JAMA Health Forum 3, e222419 (2022).
Yoon, Y. E., Kim, S. & Chang, H.-J. Artificial intelligence and echocardiography. J. Cardiovasc. Imaging 29, 193–204 (2021).
Seto, E. Y. et al. Patterns of intestinal schistosomiasis among mothers and young children from Lake Albert, Uganda: water contact and social networks inferred from wearable global positioning system dataloggers. Geospat. Health 7, 1–13 (2012).
Ozella, L. et al. Using wearable proximity sensors to characterize social contact patterns in a village of rural Malawi. EPJ Data Sci. 10, 46 (2021).
Evans, G. F., Shirk, A., Muturi, P. & Soliman, E. Z. Feasibility of using mobile ECG recording technology to detect atrial fibrillation in low-resource settings. Glob. Heart 12, 285–289 (2017).
Hughes, C. M. L. et al. Development of a post-stroke upper limb rehabilitation wearable sensor for use in sub-Saharan Africa: a pilot validation study. Front. Bioeng. Biotechnol. 7, 322 (2019).
Kim, J. et al. Skin-interfaced wireless biosensors for perinatal and paediatric health. Nat. Rev. Bioeng. 1, 631–647 (2023).
Bioengineering for low-resource settings. Nat. Rev. Bioeng. 1, 607 (2023).
Huhn, S. et al. Using wearable devices to generate real-world, individual-level data in rural, low-resource contexts in Burkina Faso, Africa: a case study. Front. Public Health 10, 972177 (2022).
Mashamba-Thompson, T. P., Pfavayi, L. T. & Mutapi, F. Blind spots in the implementation of point-of-care diagnostics for underserved communities. Nat. Rev. Bioeng. https://doi.org/10.1038/s44222-023-00127-4 (2023).
Hui, C. Y. et al. Mapping national information and communication technology (ICT) infrastructure to the requirements of potential digital health interventions in low- and middle-income countries. J. Glob. Health 12, 04094 (2022).
Shirzaei Sani, E. et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 9, eadf7388 (2023).
Xu, Y. et al. In-ear integrated sensor array for the continuous monitoring of brain activity and of lactate in sweat. Nat. Biomed. Eng. 7, 1307–1320 (2023).
Imani, S. et al. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 7, 11650 (2016).
Pu, Z. et al. A thermal activated and differential self-calibrated flexible epidermal biomicrofluidic device for wearable accurate blood glucose monitoring. Sci. Adv. 7, eabd0199 (2021).
Güder, F. et al. Paper-based electrical respiration sensor. Angew. Chem. Int. Ed. 55, 5727–5732 (2016).
Alshabouna, F. et al. PEDOT:PSS-modified cotton conductive thread for mass manufacturing of textile-based electrical wearable sensors by computerized embroidery. Mater. Today 59, 56–67 (2022).
Bandodkar Amay, J. et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci. Adv. 5, eaav3294 (2019).
Olenik, S., Lee, H. S. & Güder, F. The future of near-field communication-based wireless sensing. Nat. Rev. Mater. 6, 286–288 (2021).
Nyein, H. Y. Y. et al. Regional and correlative sweat analysis using high-throughput microfluidic sensing patches toward decoding sweat. Sci. Adv. 5, eaaw9906 (2019).
Baker, L. B. et al. Skin-interfaced microfluidic system with machine learning-enabled image processing of sweat biomarkers in remote settings. Adv. Mater. Technol. 7, 2200249 (2022).
Ghaffari, R. et al. Soft wearable systems for colorimetric and electrochemical analysis of biofluids. Adv. Funct. Mater. 30, 1907269 (2020).
Song, Y., Mukasa, D., Zhang, H. & Gao, W. Self-powered wearable biosensors. Acc. Mater. Res. 2, 184–197 (2021).
Min, J. et al. An autonomous wearable biosensor powered by a perovskite solar cell. Nat. Electron. 6, 630–641 (2023).
Yin, L. et al. A self-sustainable wearable multi-modular e-textile bioenergy microgrid system. Nat. Commun. 12, 1542 (2021).
Davis, N., Heikenfeld, J., Milla, C. & Javey, A. The challenges and promise of sweat sensing. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-02059-1 (2024).
Baker, L. B. Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature 6, 211–259 (2019).
Kamodyova, N. et al. Blood contamination in saliva: impact on the measurement of salivary oxidative stress markers. Dis. Markers 2015, 479251 (2015).
Kang, J.-H. & Kho, H.-S. Blood contamination in salivary diagnostics: current methods and their limitations. Clin. Chem. Lab. Med. 57, 1115–1124 (2019).
Cruickshank-Quinn, C. et al. Determining the presence of asthma-related molecules and salivary contamination in exhaled breath condensate. Respir. Res. 18, 57 (2017).
Rufo, J., Zhang, P., Zhong, R., Lee, L. P. & Huang, T. J. A sound approach to advancing healthcare systems: the future of biomedical acoustics. Nat. Commun. 13, 3459 (2022).
Martin, L., Hutchens, M., Hawkins, C. & Radnov, A. How much do clinical trials cost? Nat. Rev. Drug Discov. 16, 381–382 (2017).
Song, Y. et al. 3D-printed epifluidic electronic skin for machine learning–powered multimodal health surveillance. Sci. Adv. 9, eadi6492 (2023).
Yang, D. S. et al. 3D-printed epidermal sweat microfluidic systems with integrated microcuvettes for precise spectroscopic and fluorometric biochemical assays. Mater. Horiz. 10, 4992–5003 (2023).
Soto, R. J., Hall, J. R., Brown, M. D., Taylor, J. B. & Schoenfisch, M. H. In vivo chemical sensors: role of biocompatibility on performance and utility. Anal. Chem. 89, 276–299 (2017).
Hu, C., Wang, L., Liu, S., Sheng, X. & Yin, L. Recent development of implantable chemical sensors utilizing flexible and biodegradable materials for biomedical applications. ACS Nano 18, 3969–3995 (2024).
Brasier, N. & Eckstein, J. Sweat as a source of next-generation digital biomarkers. Digit. Biomark. 3, 155–165 (2019).
Gupta, N., Fischer, A. R. H. & Frewer, L. J. Socio-psychological determinants of public acceptance of technologies: a review. Public Understand. Sci. 21, 782–795 (2011).
Stein, H. F. Rehabilitation and chronic illness in American culture. J. Psychol. Anthr. 2, 153–176 (1979).
Luborsky, M. R. Sociocultural factors shaping technology usage: fulfilling the promise. Technol. Disabil. 2, 71–78 (1993).
Mushi, A. K. et al. Acceptability of malaria rapid diagnostic tests administered by village health workers in Pangani District, North eastern Tanzania. Malar. J. 15, 439 (2016).
Ngowi, K. et al. “I wish to continue receiving the reminder short messaging service”: a mixed methods study on the acceptability of digital adherence tools among adults living with HIV on antiretroviral treatment in Tanzania. Patient Prefer. Adherence 15, 559–568 (2021).
Shehada, N. et al. Silicon nanowire sensors enable diagnosis of patients via exhaled breath. ACS Nano 10, 7047–7057 (2016).
Acciaroli, G., Vettoretti, M., Facchinetti, A. & Sparacino, G. Toward calibration-free continuous glucose monitoring sensors: Bayesian calibration approach applied to next-generation dexcom technology. Diabetes Technol. Ther. 20, 59–67 (2018).
Shan, B. et al. Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano 14, 12125–12132 (2020).
Nakhleh, M. K. et al. Artificially intelligent nanoarray for the detection of preeclampsia under real-world clinical conditions. Adv. Mater. Technol. 1, 1600132 (2016).
Jackson, M. & Castle, J. R. Where do we stand with closed-loop systems and their challenges? Diabetes Technol. Ther. 22, 485–491 (2020).
Kalasin, S., Sangnuang, P. & Surareungchai, W. Lab-on-eyeglasses to monitor kidneys and strengthen vulnerable populations in pandemics: machine learning in predicting serum creatinine using tear creatinine. Anal. Chem. 93, 10661–10671 (2021).
Yang, Y. et al. Artificial intelligence-enabled detection and assessment of Parkinson’s disease using nocturnal breathing signals. Nat. Med. 28, 2207–2215 (2022).
Bashir, A. et al. Machine learning guided aptamer refinement and discovery. Nat. Commun. 12, 2366 (2021).
Sotirakis, C. et al. Identification of motor progression in Parkinson’s disease using wearable sensors and machine learning. npj Parkinsons Dis. 9, 142 (2023).
Porumb, M., Stranges, S., Pescapè, A. & Pecchia, L. Precision medicine and artificial intelligence: a pilot study on deep learning for hypoglycemic events detection based on ECG. Sci. Rep. 10, 170 (2020).
Dunn, J. et al. Wearable sensors enable personalized predictions of clinical laboratory measurements. Nat. Med. 27, 1105–1112 (2021).
Cammarota, G. et al. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat. Rev. Gastroenterol. Hepatol. 17, 635–648 (2020).
Acknowledgements
N.B. acknowledges an Early-career Fellowship from Collegium Helveticum, Zurich (CH) and a MedLab Fellowship from ETH Zurich (CH). C.D. acknowledges the OrChESTRA project from the European Union’s Horizon Europe’s research and innovation programme under grant agreement number 101079473. The language editing was conducted by A. Curtis.
Author information
Authors and Affiliations
Contributions
All authors contributed to writing and reviewing the paper. Specifically, N.B., J.W., C.D. and F.G. planned and outlined the paper. N.B. drafted the figures. W.G., J.W., C.D. and H.C.A. contributed to section ‘Wearable devices for body-fluid analysis’. I.S., J.G., N.B., N.R., M.W. and S.M. contributed to section ‘Translation and healthcare implementation’. J.R.S., F.G., S.O., E.V., D.S., R.G. and J.A.R. contributed to the section ‘The environment required for mainstream adoption’. All authors contributed to editing and finalizing the paper.
Corresponding author
Ethics declarations
Competing interests
R.G. is co-founder and CEO of Epicore Biosystems. J.W. is founder and chief scientific officer at Persperion. W.G. is co-founder and advisor at Persperity Health. E.V. serves in the ethics advisory panel of Merck AG and in the ethics advisory panel of IQVIA. J.A.R. is a co-founder and advisor to Sibel Health, Sonica and Epicore Biosystems, and holds patents associated with these companies. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Sam Emaminejad, Chwee Teck Lim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brasier, N., Wang, J., Gao, W. et al. Applied body-fluid analysis by wearable devices. Nature 636, 57–68 (2024). https://doi.org/10.1038/s41586-024-08249-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41586-024-08249-4
This article is cited by
-
Next-generation biosensing for in situ monitoring
Nature Sensors (2026)
-
Smart Textiles with Living Interfaces: Microbiome–Electronics Integration for Advanced Skin Health Management
Advanced Fiber Materials (2026)
-
Revolutionizing healthcare: the next generation of wearable chemical sensors for personal health monitoring
Science China Materials (2026)
-
Smart fiber photodetectors based on inorganic semiconductors
Science China Materials (2026)
-
Recent Advances and Prospects of MXene Fiber-Based Wearable Supercapacitors: Methods, Design, Applications to Beyond
Advanced Fiber Materials (2026)