Abstract
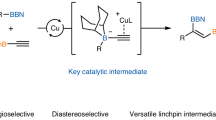
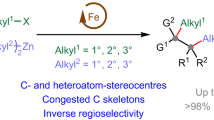
Cross-coupling of aryl boronic esters forms a cornerstone of how chemists make molecules. More recently, enantiomerically enriched boronic esters have shown great promise in modular synthesis as versatile building blocks for the rapid construction of diverse molecules. A significant challenge in this area is to employ boronic esters for the catalytic construction of C(sp3)–C(sp3) bonds, especially those where the reaction site is a stereogenic carbon center. Addressing this challenge would not only expand the utility of boronic esters in the modular synthesis of organic frameworks, but also prove more broadly beneficial in the synthesis of natural products and bioactive molecules.1 In this connection, we have developed a stereospecific C(sp3)–C(sp3) coupling reaction catalyzed by a copper acetylide complex. This reaction operates with four-coordinate boron “ate” complexes while remaining inert to simple functional groups including boronic esters, and thereby enables efficient strategies for modular synthesis of complex molecules. Applications to the synthesis of (–)-spongidepsin and the carbon skeleton of fluvirucinine A1 are described.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This Supplementary Information file contains the following sections: General Information; Experimental Section; Stereochemistry Study; Computational Details; References; and NMR Spectral Data.
Rights and permissions
About this article
Cite this article
Zhang, X., Palka, K.T., Zhang, M. et al. Stereospecific alkyl–alkyl cross-coupling of boronic esters. Nature (2026). https://doi.org/10.1038/s41586-026-10261-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-026-10261-9