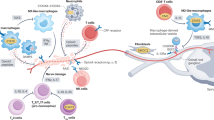
Abstract
Immune cells are involved in the pathogenesis of pain by directly activating or sensitizing nociceptor sensory neurons. However, because the immune system also has the capacity to self-regulate through anti-inflammatory mechanisms that drive the resolution of inflammation, it might promote pain resolution and prevention. Here, we describe how immune cell-derived cytokines can act directly on sensory neurons to inhibit pain hypersensitivity and how immune-derived endogenous opioids promote analgesia. We also discuss how immune cells support healthy tissue innervation by clearing debris after nerve injury, protecting against axon retraction from target tissues and enhancing regeneration, preventing the development of chronic neuropathic pain. Finally, we review the accumulating evidence that manipulating immune activity positively alters somatosensation, albeit with currently unclear molecular and cellular mechanisms. Exploration of immune-mediated analgesia and pain prevention could, therefore, be important for the development of novel immune therapies for the treatment of clinical pain states.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jain, A., Hakim, S. & Woolf, C. J. Immune drivers of physiological and pathological pain. J. Exp. Med. 221, e20221687 (2024).
Marchand, F., Perretti, M. & McMahon, S. B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 6, 521–532 (2005).
Yang, J. X. et al. Potential neuroimmune interaction in chronic pain: a review on immune cells in peripheral and central sensitization. Front. Pain. Res. 3, 946846 (2022).
Talbot, S., Foster, S. L. & Woolf, C. J. Neuroimmunity: physiology and pathology. Annu. Rev. Immunol. https://doi.org/10.1146/annurev-immunol-041015-055340 (2016).
Decosterd, I. & Woolf, C. J. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158 (2000).
Alvarez, P., Bogen, O., Green, P. G. & Levine, J. D. Nociceptor interleukin 10 receptor 1 is critical for muscle analgesia induced by repeated bouts of eccentric exercise in the rat. Pain 158, 1481–1488 (2017).
Laumet, G. et al. Interleukin-10 resolves pain hypersensitivity induced by cisplatin by reversing sensory neuron hyperexcitability. Pain 161, 2344–2352 (2020).
Sun, Q. et al. IRG1/itaconate increases IL-10 release to alleviate mechanical and thermal hypersensitivity in mice after nerve injury. Front. Immunol. 13, 1012442 (2022).
Üçeyler, N., Topuzoǧlu, T., Schießer, P., Hahnenkamp, S. & Sommer, C. IL-4 deficiency is associated with mechanical hypersensitivity in mice. PLoS ONE 6, e28205 (2011).
Celik, M., Labuz, D., Keye, J., Glauben, R. & Machelska, H. IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight 5, e133093 (2020).
Prado, J. et al. Cytokine receptor clustering in sensory neurons with an engineered cytokine fusion protein triggers unique pain resolution pathways. Proc. Natl Acad. Sci. USA 118, e2009647118 (2021).
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Xu, Z. Z. et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 16, 592–597 (2010).
Wu, J. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830 (2013).
Defaye, M. et al. Induction of antiviral interferon-stimulated genes by neuronal STING promotes the resolution of pain in mice. J. Clin. Invest. 134, e176474 (2024).
Donnelly, C. R. et al. STING controls nociception via type I interferon signalling in sensory neurons. Nature 591, 275–280 (2021). In this study, the authors found that neuron-intrinsic innate immune signaling via the IFN–STING pathway is important for controlling nociception in mice, and exogenous activation of this pathway suppressed excitability of nociceptors and reduced pain thresholds.
Wang, K. et al. STING suppresses bone cancer pain via immune and neuronal modulation. Nat. Commun. 12, 4558 (2021).
Binshtok, A. M. et al. Nociceptors are interleukin-1β sensors. J. Neurosci. 28, 14062 (2008).
Liu, X. J. et al. Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 24, 1374–1377 (2014).
Jain, A. et al. Nociceptor-immune interactomes reveal insult-specific immune signatures of pain. Nat. Immunol. 25, 1296–1305 (2024).
Kim, J. H., Park, J. S. & Park, D. Anti-allodynic effect of interleukin 10 in a mouse model of complex regional pain syndrome through reduction of NK1 receptor expression of microglia in the spinal cord. J. Pain. Res. 11, 1729–1741 (2018).
Mitsui, K. et al. Role of macrophage autophagy in postoperative pain and inflammation in mice. J. Neuroinflammation 20, 102 (2023).
de Souza, S. et al. Mast cell-derived chymases are essential for the resolution of inflammatory pain in mice. Preprint at bioRxiv https://doi.org/10.1101/2024.08.05.606617 (2024).
Starkl, P. et al. Mast cell-derived BH4 and serotonin are critical mediators of postoperative pain. Sci. Immunol. 9, 98 (2024).
Van Der Vlist, M. et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron https://doi.org/10.1016/j.neuron.2021.11.020 (2022).
Fischer, R. et al. TNFR2 promotes Treg-mediated recovery from neuropathic pain across sexes. Proc. Natl Acad. Sci. USA 116, 17045–17050 (2019).
Hu, R., Zhang, J., Liu, X., Huang, D. & Cao, Y. Q. Low-dose interleukin-2 and regulatory T cell treatments attenuate punctate and dynamic mechanical allodynia in a mouse model of sciatic nerve injury. J. Pain. Res. 14, 893–906 (2021).
Austin, P. J., Kim, C. F., Perera, C. J. & Moalem-Taylor, G. Regulatory T cells attenuate neuropathic pain following peripheral nerve injury and experimental autoimmune neuritis. Pain 153, 1916–1931 (2012).
Laumet, G., Edralin, J. D., Dantzer, R., Heijnen, C. J. & Kavelaars, A. Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain 160, 1459–1468 (2019).
Singh, S. K. et al. CD8+ T cell-derived IL-13 increases macrophage IL-10 to resolve neuropathic pain. JCI Insight 7, e154194 (2022).
Parisien, M. et al. Genome-wide association studies with experimental validation identify a protective role for B lymphocytes against chronic post-surgical pain. Br. J. Anaesth. 133, 360–370 (2024).
Parisien, M. et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci. Transl. Med. 14, eabj9954 (2022). In individuals with resolved low back pain, there was an enrichment in neutrophil gene signatures compared to those with persistent pain, suggesting neutrophils promote resolution of pain. This was further shown in mice where administration of neutrophils promoted the resolution of inflammatory pain. In addition, the data in this study showed that the chronic use of nonsteroidal anti-inflammatory drugs was associated with persistent pain, which was recapitulated in animals.
Corder, G., Castro, D. C., Bruchas, M. R. & Scherrer, G. Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci. 41, 453–473 (2018).
Sobczak, M., Sałaga, M., Storr, M. A. & Fichna, J. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J. Gastroenterol. 49, 24–45 (2014).
Cabot, P. J., Carter, L., Schäfer, M. & Stein, C. Methionine-enkephalin- and dynorphin A-release from immune cells and control of inflammatory pain. Pain 93, 207–212 (2001).
Pannell, M. et al. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J. Neuroinflammation 13, 262 (2016).
Shi, J. T. et al. Local analgesia of electroacupuncture is mediated by the recruitment of neutrophils and released β-endorphins. Pain 164, 1965–1975 (2023).
Wu, H. Y. et al. Spinal interleukin-10 produces antinociception in neuropathy through microglial β-endorphin expression, separated from antineuroinflammation. Brain Behav. Immun. 73, 504–519 (2018).
Wu, H. Y., Tang, X. Q., Mao, X. F. & Wang, Y. X. Autocrine interleukin-10 mediates glucagon-like peptide-1 receptor-induced spinal microglial β-endorphin expression. J. Neurosci. 37, 11701–11714 (2017).
Wu, H. Y., Mao, X. F., Fan, H. & Wang, Y. X. p38 β mitogen-activated protein kinase signaling mediates exenatide-stimulated microglial β-endorphin expression. Mol. Pharmacol. 91, 451–463 (2017).
Apryani, E. et al. The spinal microglial IL-10/β-endorphin pathway accounts for cinobufagin-induced mechanical antiallodynia in bone cancer pain following activation of α7-nicotinic acetylcholine receptors. J. Neuroinflammation 17, 75 (2020).
Han, Q. Q. et al. Cynandione A alleviates neuropathic pain through α7-nAChR-dependent IL-10/β-endorphin signaling complexes. Front. Pharm. 11, 614450 (2021).
Basso, L. et al. Mobilization of CD4+ T lymphocytes in inflamed mucosa reduces pain in colitis mice: toward a vaccinal strategy to alleviate inflammatory visceral pain. Pain 159, 331–341 (2018).
Rosen, S. F. et al. T-cell mediation of pregnancy analgesia affecting chronic pain in mice. J. Neurosci. 37, 9819–9827 (2017).
Rosen, S. F. et al. Increased pain sensitivity and decreased opioid analgesia in T-cell-deficient mice and implications for sex differences. Pain 160, 358–366 (2019).
Midavaine, É. et al. Regulatory T cell-derived enkephalin imparts pregnancy-induced analgesia. Preprint at bioRxiv https://doi.org/10.1101/2024.05.11.593442 (2024).
Starowicz, K. & Finn, D. P. Cannabinoids and pain: sites and mechanisms of action. Adv. Pharm. 80, 437–475 (2017).
Finn, D. P. et al. Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain 162, S5–S25 (2021).
Sagar, D. R. et al. Endocannabinoid regulation of spinal nociceptive processing in a model of neuropathic pain. Eur. J. Neurosci. 31, 1414–1422 (2010).
Xu, J. et al. Activation of cannabinoid receptor 2 attenuates mechanical allodynia and neuroinflammatory responses in a chronic post-ischemic pain model of complex regional pain syndrome type I in rats. Eur. J. Neurosci. 44, 3046–3055 (2016).
Xu, J. J. et al. Spinal gene expression profiling and pathways analysis of a CB2 agonist (MDA7)-targeted prevention of paclitaxel-induced neuropathy. Neuroscience 260, 185–194 (2014).
Wu, J., Hocevar, M., Bie, B., Foss, J. F. & Naguib, M. Cannabinoid type 2 receptor system modulates paclitaxel-induced microglial dysregulation and central sensitization in rats. J. Pain. 20, 501–514 (2019).
Carrier, E. J. et al. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol. Pharmacol. 65, 999–1007 (2004).
Ydens, E. et al. Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat. Neurosci. 23, 676–689 (2020).
Lindborg, J. A., Mack, M. & Zigmond, R. E. Neutrophils are critical for myelin removal in a peripheral nerve injury model of Wallerian degeneration. J. Neurosci. 37, 10258–10277 (2017). While macrophages were thought to be required for myelin clearance following nerve injury, this study showed that macrophages were not required and that, instead, neutrophils are capable of compensating for lack of macrophage infiltration and are in fact necessary for proper myelin removal and, therefore, proper regeneration following injury.
Talsma, A. D., Niemi, J. P. & Zigmond, R. E. Neither injury induced macrophages within the nerve, nor the environment created by Wallerian degeneration is necessary for enhanced in vivo axon regeneration after peripheral nerve injury. J. Neuroinflammation 21, 134 (2024).
Stratton, J. A. et al. Macrophages regulate Schwann cell maturation after nerve injury. Cell Rep. https://doi.org/10.1016/j.celrep.2018.08.004 (2018).
Niehaus, J. K., Taylor-Blake, B., Loo, L., Simon, J. M. & Zylka, M. J. Spinal macrophages resolve nociceptive hypersensitivity after peripheral injury. Neuron 109, 1274–1282.e6 (2021).
Hakim, S. et al. Macrophages protect against sensory axon degeneration in diabetic neuropathy. Preprint at bioRxiv https://doi.org/10.1101/2024.01.30.577801 (2024).
Davies, A. J. et al. Natural killer cells degenerate intact sensory afferents following nerve injury article natural killer cells degenerate intact sensory afferents following nerve injury. Cell 176, 716–728 (2019). In this study, the authors found that NK cells infiltrate damaged sciatic nerves following injury and recognize injured axons by binding to RAE1 via NKG2D. NK cells are then critical for the clearance of damaged axons, allowing functional regeneration, and preventing the development of chronic pain.
Renthal, W. et al. Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron 108, 128–144 (2020).
Feng, R., Saraswathy, V. M., Mokalled, M. H. & Cavalli, V. Self-renewing macrophages in dorsal root ganglia contribute to promote nerve regeneration. Proc. Natl Acad. Sci. USA 120, e2215906120 (2023).
Kwon, M. J. et al. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J. Neurosci. 33, 15095–15108 (2013).
Jha, M. K. et al. Macrophage monocarboxylate transporter 1 promotes peripheral nerve regeneration after injury in mice. J. Clin. Invest. 131, e141964 (2021).
Enamorado, M. et al. Immunity to the microbiota promotes sensory neuron regeneration. Cell 186, 607–620 (2023).
Jerome, A. D. et al. Cytokine polarized, alternatively activated bone marrow neutrophils drive axon regeneration. Nat. Immunol. 25, 957–968 (2024).
Wang, X. et al. Driving axon regeneration by orchestrating neuronal and non-neuronal innate immune responses via the IFNγ-cGAS-STING axis. Neuron 111, 236–255 (2023).
Gangadharan, V. et al. Neuropathic pain caused by miswiring and abnormal end organ targeting. Nature 606, 137–145 (2022).
Kupari, J. & Ernfors, P. Molecular taxonomy of nociceptors and pruriceptors. Pain 164, 1245–1257 (2023).
Qi, L. et al. A mouse DRG genetic toolkit reveals morphological and physiological diversity of somatosensory neuron subtypes. Cell 187, 1508–1526 (2024).
Megat, S. et al. Differences between dorsal root and trigeminal ganglion nociceptors in mice revealed by translational profiling. J. Neurosci. 39, 6829–6847 (2019).
O’Brien, D. E., Brenner, D. S., Gutmann, D. H. & Gereau, R. W. IV Assessment of pain and itch behavior in a mouse model of neurofibromatosis type 1. J. Pain. 14, 628–637 (2013).
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010).
Drewes, A. M. et al. Gastrointestinal pain. Nat. Rev. Dis. Primers 6, 1 (2020).
Acknowledgements
This study was funded by National Institutes of Health (NIH) grants R35 NS105076 to C.J.W., F31NS127357 to S.H. and 1K99AR083482-01 to A.J.
Author information
Authors and Affiliations
Contributions
S.H. and A.J. equally contributed to the conceptualization and writing of this manuscript. C.J.W. supervised the organization and scope of the review and contributed to its writing.
Corresponding author
Ethics declarations
Competing interests
C.J.W. is a founder of Nocion Therapeutics, Quralis and Blackbox Bio, and a Scientific Advisory Board member of Lundbeck, Axonis and Tafalgie Therapeutics. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Brian Kim, De’Broski Herbert, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ioana Staicu, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hakim, S., Jain, A. & Woolf, C.J. Immune drivers of pain resolution and protection. Nat Immunol 25, 2200–2208 (2024). https://doi.org/10.1038/s41590-024-02002-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-024-02002-9
This article is cited by
-
Ectopic Nociceptor Sprouting as a Key Peripheral Driver of Pain in Rheumatoid Arthritis
Current Rheumatology Reports (2025)