Abstract
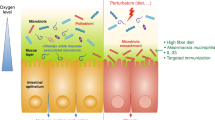
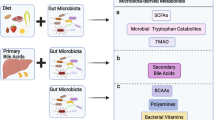
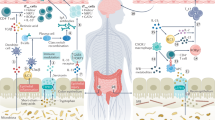
The microbiota has a fundamental role in regulating homeostasis and inflammation across the barrier surfaces of the body. The gut is a unique bioreactor where the high concentration of microorganisms, microbial and dietary metabolites, microbial-derived molecular structures, immune cells, stroma and neurons form a complex, highly interactive and precisely regulated system. The mucosal immune system in the gut has profound local and systemic effects, influencing both health and disease. A critical period of immune imprinting occurs early in life, shaped by the neonatal microbiota and nutrition, to influence immune development and long-term disease susceptibility. Microbiota-derived metabolites have crucial roles in immune modulation, influencing epithelial integrity, oral tolerance and inflammatory responses. This Review explores the interactions between the microbiota and the mucosal immune system from infancy to adulthood, highlighting the impact on health and disease. We also discuss therapeutic interventions, including microbiota-derived molecules, dietary metabolites and emerging microbiome-based co-therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014).
Healy, D. B. Clinical implications of preterm infant gut microbiome development. Nat. Microbiol. 7, 22–33 (2022).
Dhariwala, M. O. & Scharschmidt, T. C. Baby’s skin bacteria: first impressions are long-lasting. Trends Immunol. 42, 1088–1099 (2021).
Gensollen, T. How colonization by microbiota in early life shapes the immune system. Science 352, 539–544 (2016).
Bain, C. C. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 15, 929–937 (2014).
Ennamorati, M. Intestinal microbes influence development of thymic lymphocytes in early life. Proc. Natl Acad. Sci. USA 117, 2570–2578 (2020).
Suo, C. Mapping the developing human immune system across organs. Science 376, eabo0510 (2022).
Connors, T. J. Site-specific development and progressive maturation of human tissue-resident memory T cells over infancy and childhood. Immunity 56, 1894–1909 (2023).
Aversa, Z. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin. Proc. 96, 66–77 (2021).
Kronman, M. P. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 130, 794–803 (2012).
Azad, M. B. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int. J. Obes. 38, 1290–1298 (2014).
Kennedy, K. M. et al. Questioning the fetal microbiome illustrates pitfalls of low-biomass microbial studies. Nature 613, 639–649 (2023).
Husso, A. et al. Impacts of maternal microbiota and microbial metabolites on fetal intestine, brain, and placenta. BMC Biol. 21, 207 (2023).
Pessa-Morikawa, T. et al. Maternal microbiota-derived metabolic profile in fetal murine intestine, brain and placenta. BMC Microbiol. 22, 46 (2022).
Li, N. et al. Memory CD4+ T cells are generated in the human fetal intestine. Nat. Immunol. 20, 301–312 (2019).
Mishra, A. et al. Microbial exposure during early human development primes fetal immune cells. Cell 184, 3394–3409 (2021).
Gomez de Agüero, M. et al. The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302 (2016).
Lim, A. I. et al. Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Science 373, eabf3002 (2021).
Singhal, R. & Shah, Y. M. Oxygen battle in the gut: hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 295, 10493–10505 (2020).
Sanidad, K. Z. & Zeng, M. Y. Neonatal gut microbiome and immunity. Curr. Opin. Microbiol. 56, 30–37 (2020).
Chong, C. Y. L., Bloomfield, F. H. & O’Sullivan, J. M. Factors affecting gastrointestinal microbiome development in neonates. Nutrients 10, 274 (2018).
Al Nabhani, Z. A weaning reaction to microbiota is required for resistance to immunopathologies in the adult. Immunity 50, 1276–1288 (2019).
Shao, Y. et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574, 117–121 (2019).
Vatanen, T. et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165, 842–853 (2016).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015).
Schaupp, L. et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells. Cell 181, 1080–1096 (2020).
Abt, M. C. et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170 (2012).
Bain, C. C. & Mowat, A. M. Macrophages in intestinal homeostasis and inflammation. Immunol. Rev. 260, 102–117 (2014).
Chang, P. V., Hao, L., Offermanns, S. & Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl Acad. Sci. USA 111, 2247–2252 (2014).
Singh, D. K. Necrotizing enterocolitis: bench to bedside approaches and advancing our understanding of disease pathogenesis. Front. Pediatr. 10, 1107404 (2022).
Aziz, M., Prince, J. M. & Wang, P. Gut microbiome and necrotizing enterocolitis: understanding the connection to find a cure. Cell Host Microbe 30, 612–616 (2022).
Hunter, C. J. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr. Res. 63, 117–123 (2008).
Singer, J. R. Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis. Nat. Med. 25, 1772–1782 (2019).
Lubin, J. B. Arresting microbiome development limits immune system maturation and resistance to infection in mice. Cell Host Microbe 31, 554–570 7 (2023).
Kim, Y. G. Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens. Science 356, 315–319 (2017).
Koch, M. A. Maternal IgG and IgA antibodies dampen mucosal T helper cell responses in early life. Cell 165, 827–841 (2016).
Sanidad, K. Z. Maternal gut microbiome-induced IgG regulates neonatal gut microbiome and immunity. Sci. Immunol. 7, 3816 (2022).
Johnson-Hence, C. B. Stability and heterogeneity in the antimicrobiota reactivity of human milk-derived immunoglobulin A. J. Exp. Med. 220, e20220839 (2023).
Hild, B., Dreier, M. S. & Oh, J. H. Neonatal exposure to a wild-derived microbiome protects mice against diet-induced obesity. Nat. Metab. 3, 1042–1057 (2021).
Leonardi, I. Mucosal fungi promote gut barrier function and social immunity. Cell 185, 831–846 (2022).
Hill, J. H. et al. Neonatal fungi promote lifelong metabolic health through macrophage-dependent β cell development. Science 387, eadn0953 (2025).
Nava, P. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity 32, 392–402 (2010).
Sefik, E. Mucosal immunology. Individual intestinal symbionts induce a distinct population of RORγ+ regulatory T cells. Science 349, 993–997 (2015).
Xu, M. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554, 373–377 (2018).
Hepworth, M. R. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature 498, 113–117 (2013).
Hepworth, M. R. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science 348, 1031–1035 (2015).
Lyu, M. et al. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature 610, 744–751 (2022).
Kedmi, R. A RORγt+ cell instructs gut microbiota-specific Treg cell differentiation. Nature 610, 737–743 (2022).
Akagbosu, B. Novel antigen-presenting cell imparts Treg-dependent tolerance to gut microbiota. Nature 610, 752–760 (2022).
Fu, L. et al. PRDM16-dependent antigen-presenting cells induce tolerance to gut antigens. Nature 642, 756–765 (2025).
Zhou, W. et al. ILC3s sense gut microbiota through STING to initiate immune tolerance. Immunity 58, 1762–1777.e7 (2025).
Ulezko Antonova, A. et al. A distinct human cell type expressing MHCII and RORγt with dual characteristics of dendritic cells and type 3 innate lymphoid cells. Proc. Natl Acad. Sci. USA 120, e2318710120 (2023).
Rodrigues, P. F. et al. Rorγt-positive dendritic cells are required for the induction of peripheral regulatory T cells in response to oral antigens. Cell 188, 2720–2737 (2025).
Rankin, L. C. Dietary tryptophan deficiency promotes gut RORγt+ Treg cells at the expense of Gata3+ Treg cells and alters commensal microbiota metabolism. Cell Rep. 42, 112135 (2023).
Cerovic, V., Pabst, O. & Mowat, A. M. The renaissance of oral tolerance: merging tradition and new insights. Nat. Rev. Immunol. 25, 42–56 (2025).
Matteoli, G. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut 59, 595–604 (2010).
Kulkarni, D. H. Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal Immunol. 13, 271–282 (2020).
Sanidad, K. Z. Gut bacteria-derived serotonin promotes immune tolerance in early life. Sci. Immunol. 9, eadj4775 (2024).
Henrick, B. M. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898 (2021).
Veenbergen, S. Colonic tolerance develops in the iliac lymph nodes and can be established independent of CD103+ dendritic cells. Mucosal Immunol. 9, 894–906 (2016).
Canesso, M. C. C. Identification of antigen-presenting cell–T cell interactions driving immune responses to food. Science 387, eado5088 (2024).
Sun, I. -H. et al. RORγt eTACs mediate oral tolerance and Treg induction. J. Exp. Med. 222, e20250573 (2025).
Cabric, V. et al. A wave of Thetis cells imparts tolerance to food antigens early in life. Science 389, 268–274 (2025).
Teng, F. et al. ILC3s control airway inflammation by limiting T cell responses to allergens and microbes. Cell Rep. 37, 110051 (2021).
Hansson, G. C. Mucins and the microbiome. Annu. Rev. Biochem. 89, 769–793 (2020).
Paone, P. & Cani, P. D. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut 69, 2232–2243 (2020).
Johansson, M. E. V. et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 18, 582–592 (2015).
Naama, M. et al. Autophagy controls mucus secretion from intestinal goblet cells by alleviating ER stress. Cell Host Microbe 31, 433–446 (2023).
Ramanan, D. & Cadwell, K. Intrinsic defense mechanisms of the intestinal epithelium. Cell Host Microbe 19, 434–441 (2016).
Santos-Júnior, C. D. et al. Discovery of antimicrobial peptides in the global microbiome with machine learning. Cell 187, 3761–3778 (2024).
Macpherson, A. J. & McCoy, K. D. Stratification and compartmentalisation of immunoglobulin responses to commensal intestinal microbes. Semin. Immunol. 25, 358–363 (2013).
Doron, I. et al. Mycobiota-induced IgA antibodies regulate fungal commensalism in the gut and are dysregulated in Crohn’s disease. Nat. Microbiol. 6, 1493–1504 (2021).
Blander, J. M. On cell death in the intestinal epithelium and its impact on gut homeostasis. Curr. Opin. Gastroenterol. 34, 413–419 (2018).
Cummings, R. J. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature 539, 565–569 (2016).
Ghazavi, F. Executioner caspases 3 and 7 are dispensable for intestinal epithelium turnover and homeostasis at steady state. Proc. Natl Acad. Sci. USA 119, e2024508119 (2022).
Lawlor, K. E., Murphy, J. M. & Vince, J. E. Gasdermin and MLKL necrotic cell death effectors: signaling and diseases. Immunity 57, 429–445 (2024).
Zindel, J. & Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. 15, 493–518 (2020).
Kayagaki, N. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021).
Bulek, K. Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis. J. Clin. Invest. 130, 4218–4234 (2020).
Evavold, C. L. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44 (2018).
Boyapati, R. K., Rossi, A. G., Satsangi, J. & Ho, G. T. Gut mucosal DAMPs in IBD: from mechanisms to therapeutic implications. Mucosal Immunol. 9, 567–582 (2016).
Chiou, S. An immunohistochemical atlas of necroptotic pathway expression. EMBO Mol. Med. 16, 1717–1749 (2024).
Patankar, J. V. & Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 17, 543–556 (2020).
Ivanov, A. I., Rana, N., Privitera, G. & Pizarro, T. T. The enigmatic roles of epithelial gasdermin B: recent discoveries and controversies. Trends Cell Biol. 33, 48–59 (2023).
Privitera, G., Rana, N., Armuzzi, A. & Pizarro, T. T. The gasdermin protein family: emerging roles in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 20, 366–387 (2023).
Soderman, J., Berglind, L. & Almer, S. Gene expression-genotype analysis implicates GSDMA, GSDMB, and LRRC3C as contributors to inflammatory bowel disease susceptibility. Biomed. Res. Int. 2015, 834805 (2015).
Rana, N. GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell 185, 283–298 217 (2022).
Tan, G., Huang, C., Chen, J., Chen, B. & Zhi, F. Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn’s disease by promoting intestinal inflammation. Cell Rep. 35, 109265 (2021).
Foerster, E. G. How autophagy controls the intestinal epithelial barrier. Autophagy 18, 86–103 (2022).
Eugene, S. P., Reddy, V. S. & Trinath, J. Endoplasmic reticulum stress and intestinal inflammation: a perilous union. Front. Immunol. 11, 543022 (2020).
Mudde, A. C. A., Booth, C. & Marsh, R. A. Evolution of our understanding of XIAP deficiency. Front. Pediatr. 9, 660520 (2021).
Gumede, D. B., Abrahamse, H. & Houreld, N. N. Targeting Wnt/β-catenin signaling and its interplay with TGF-β and Notch signaling pathways for the treatment of chronic wounds. Cell Commun. Signal 22, 244 (2024).
Cialdai, F., Risaliti, C. & Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 10, 958381 (2022).
Fullerton, J. N. & Gilroy, D. W. Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 15, 551–567 (2016).
Neurath, M. F. Resolution of inflammation: from basic concepts to clinical application. Semin. Immunopathol. 41, 627–631 (2019).
Medina, C. B. Metabolites released from apoptotic cells act as tissue messengers. Nature 580, 130–135 (2020).
Mehrotra, P. Oxylipins and metabolites from pyroptotic cells act as promoters of tissue repair. Nature 631, 207–215 (2024).
Chang, E. B. Epithelial wound healing in inflammatory bowel diseases: the next therapeutic frontier. Transl. Res. 236, 35–51 (2021).
Metwaly, A., Reitmeier, S. & Haller, D. Microbiome risk profiles as biomarkers for inflammatory and metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 19, 383–397 (2022).
Pascal, V. et al. A microbial signature for Crohn’s disease. Gut 66, 813–822 (2017).
Vich et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl. Med. 10, eaap8914 (2018).
Franzosa, E. A. et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol 4, 293–305 (2018).
Lopez-Siles, M., Duncan, S. H., Garcia-Gil, L. J. & Martinez-Medina, M. Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. ISME J. 11, 841–852 (2017).
Lenoir, M. et al. Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes 12, 1–16 (2020).
Quévrain, E. et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 65, 415–425 (2016).
Lange, O., Proczko-Stepaniak, M. & Mika, A. Short-chain fatty acids—a product of the microbiome and its participation in two-way communication on the microbiome-host mammal line. Curr. Obes. Rep. 12, 108–126 (2023).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Josefowicz, S. Z. et al. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399 (2012).
Byndloss, M. X. et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575 (2017).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019).
Machiels, K. et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63, 1275–1283 (2014).
Sorbara, M. T. et al. Inhibiting antibiotic-resistant Enterobacteriaceae by microbiota-mediated intracellular acidification. J. Exp. Med. 216, 84–98 (2019).
McCrory, C., Lenardon, M. & Traven, A. Bacteria-derived short-chain fatty acids as potential regulators of fungal commensalism and pathogenesis. Trends Microbiol. 32, 1106–1118 (2024).
van Best, N. et al. Bile acids drive the newborn’s gut microbiota maturation. Nat. Commun. 11, 3692 (2020).
Ridlon, J. M., Kang, D. -J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259 (2006).
Devlin, A. S. & Fischbach, M. A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 11, 685–690 (2015).
Thomas, J. P., Modos, D., Rushbrook, S. M., Powell, N. & Korcsmaros, T. The emerging role of bile acids in the pathogenesis of inflammatory bowel disease. Front. Immunol. 13, 829525 (2022).
Hang, S. et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148 (2019).
Paik, D. et al. Human gut bacteria produce ΤΗ17-modulating bile acid metabolites. Nature 603, 907–912 (2022).
Song, X. et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415 (2020).
Sinha, S. R. et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe 27, 659–670 (2020).
Quinn, R. A. et al. Global chemical effects of the microbiome include new bile-acid conjugations. Nature 579, 123–129 (2020).
Gentry, E. C. et al. Reverse metabolomics for the discovery of chemical structures from humans. Nature 626, 419–426 (2024).
Agus, A., Planchais, J. & Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724 (2018).
Cao, Y. et al. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science 378, eabm3233 (2022).
Schumacher, F. et al. A secondary metabolite of Brassicales, 1-methoxy-3-indolylmethyl glucosinolate, as well as its degradation product, 1-methoxy-3-indolylmethyl alcohol, forms DNA adducts in the mouse, but in varying tissues and cells. Arch. Toxicol. 88, 823–836 (2014).
Gronke, K. et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566, 249–253 (2019).
Nougayrède, J. -P. et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851 (2006).
Furuichi, M. et al. Commensal consortia decolonize Enterobacteriaceae via ecological control. Nature 633, 878–886 (2024).
Kim, M. et al. Critical role for the microbiota in CX3CR1+ intestinal mononuclear phagocyte regulation of intestinal T cell responses. Immunity 49, 151–163 (2018).
Viladomiu, M. et al. Adherent-invasive E. coli metabolism of propanediol in Crohn’s disease regulates phagocytes to drive intestinal inflammation. Cell Host Microbe 29, 607–619 (2021).
Leonardi, I. et al. CX3CR1+ mononuclear phagocytes control immunity to intestinal fungi. Science 359, 232–236 (2018).
Doron, I. et al. Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 184, 1017–1031 (2021).
Britton, G. J. et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut TH17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity 50, 212–224 (2019).
Brockmann, L. et al. Intestinal microbiota-specific TH17 cells possess regulatory properties and suppress effector T cells via c-MAF and IL-10. Immunity 56, 2719–2735 (2023).
Kawano, Y. et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell 185, 3501–3519 (2022).
Macpherson, A. J. et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288, 2222–2226 (2000).
Bunker, J. J. et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 358, eaan6619 (2017).
Ost, K. S. et al. Adaptive immunity induces mutualism between commensal eukaryotes. Nature 596, 114–118 (2021).
Arifuzzaman, M. et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature 611, 578–584 (2022).
Arifuzzaman, M. Dietary fiber is a critical determinant of pathologic ILC2 responses and intestinal inflammation. J. Exp. Med. 221, e20232148 (2024).
Cui, W. Diet-mediated constitutive induction of novel IL-4+ ILC2 cells maintains intestinal homeostasis in mice. J. Exp. Med. 220, e20221773 (2023).
Liao, Y. et al. Fungal symbiont transmitted by free-living mice promotes type 2 immunity. Nature 636, 697–704 (2024).
Horn, V. & Sonnenberg, G. F. Group 3 innate lymphoid cells in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 21, 428–443 (2024).
Spits, H. Innate lymphoid cells–a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Nash, A. K. et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 5, 153 (2017).
Human Microbiome Jumpstart Reference Strains Consortium. et al. A catalog of reference genomes from the human microbiome. Science 328, 994–999 (2010).
Auchtung, T. A. et al. Investigating colonization of the healthy adult gastrointestinal tract by fungi. mSphere 3, e00092-18 (2018).
Sokol, H. et al. Fungal microbiota dysbiosis in IBD. Gut 66, 1039–1048 (2017).
Chehoud, C. et al. Fungal signature in the gut microbiota of pediatric patients with inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1948–1956 (2015).
Hoarau, G. et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial Crohn’s disease. mBio 7, e01250-16 (2016).
Liguori, G. et al. Fungal dysbiosis in mucosa-associated microbiota of Crohn’s disease patients. J. Crohns Colitis 10, 296–305 (2016).
Ott, S. J. et al. Fungi and inflammatory bowel diseases: alterations of composition and diversity. Scand. J. Gastroenterol. 43, 831–841 (2008).
Li, X. V. et al. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 603, 672–678 (2022).
Pappas, P. G., Lionakis, M. S., Arendrup, M. C., Ostrosky-Zeichner, L. & Kullberg, B. J. Invasive candidiasis. Nat. Rev. Dis. Primers 4, 18026 (2018).
Limon, J. J. et al. Malassezia is associated with Crohn’s disease and exacerbates colitis in mouse models. Cell Host Microbe 25, 377–388 (2019).
Auchtung, T. A. et al. Temporal changes in gastrointestinal fungi and the risk of autoimmunity during early childhood: the TEDDY study. Nat. Commun. 13, 3151 (2022).
Iliev, I. D. et al. Focus on fungi. Cell 187, 5119–5482 (2024).
Lionakis, M. S., Drummond, R. A. & Hohl, T. M. Immune responses to human fungal pathogens and therapeutic prospects. Nat. Rev. Immunol. 23, 433–452 (2023).
Malamud, M. et al. Recognition and control of neutrophil extracellular trap formation by MICL. Nature 633, 442–450 (2024).
Espinosa, V. et al. Type III interferon is a critical regulator of innate antifungal immunity. Sci. Immunol. 2, eaan5357 (2017).
Mills, K. A. M. et al. GM-CSF–mediated epithelial-immune cell cross-talk orchestrates pulmonary immunity to Aspergillus fumigatus. Sci. Immunol. 10, eadr0547 (2025).
Kusakabe, T. et al. Fungal microbiota sustains lasting immune activation of neutrophils and their progenitors in severe COVID-19. Nat. Immunol. 24, 1879–1889 (2023).
Tso, G. H. W. et al. Experimental evolution of a fungal pathogen into a gut symbiont. Science 362, 589–595 (2018).
Chen, Y. -H. et al. Rewilding of laboratory mice enhances granulopoiesis and immunity through intestinal fungal colonization. Sci. Immunol. 8, eadd6910 (2023).
Aggor, F. E. Y. et al. Combinatorial actions of IL-22 and IL-17 drive optimal immunity to oral candidiasis through SPRRs. PLoS Pathog. 20, e1012302 (2024).
Pierre, J. F. et al. Peptide YY: a Paneth cell antimicrobial peptide that maintains Candida gut commensalism. Science 381, 502–508 (2023).
Aggor, F. E. Y. et al. Oral epithelial IL-22/STAT3 signaling licenses IL-17–mediated immunity to oral mucosal candidiasis. Sci. Immunol. 5, eaba0570 (2020).
Bacher, P. et al. Human anti-fungal TH17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell 176, 1340–1355 (2019).
Break, T. J. et al. Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science 371, eaay5731 (2021).
Desai, J. V. et al. C5a-licensed phagocytes drive sterilizing immunity during systemic fungal infection. Cell 186, 2802–2822 (2023).
Sekeresova Kralova, J. et al. Competitive fungal commensalism mitigates candidiasis pathology. J. Exp. Med. 221, e20231686 (2024).
Clooney, A. G. et al. Whole-virome analysis sheds light on viral dark matter in inflammatory bowel disease. Cell Host Microbe 26, 764–778 (2019).
Adiliaghdam, F. et al. Human enteric viruses autonomously shape inflammatory bowel disease phenotype through divergent innate immunomodulation. Sci. Immunol. 7, eabn6660 (2022).
Gogokhia, L. et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 25, 285–299 (2019).
Ungaro, F. et al. Metagenomic analysis of intestinal mucosa revealed a specific eukaryotic gut virome signature in early-diagnosed inflammatory bowel disease. Gut Microbes 10, 149–158 (2019).
Cadwell, K. et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell 141, 1135–1145 (2010).
Norman, J. M. et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160, 447–460 (2015).
Moltke, J., Ji, M., Liang, H. E. & Locksley, R. M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225 (2016).
Howitt, M. R. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333 (2016).
Gerbe, F. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230 (2016).
Nadjsombati, M. S. Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49, 33–41 (2018).
Schneider, C. A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 174, 271–284 (2018).
Lei, W. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc. Natl Acad. Sci. USA 115, 5552–5557 (2018).
Fung, C. Tuft cells mediate commensal remodeling of the small intestinal antimicrobial landscape. Proc. Natl Acad. Sci. USA 120, 2216908120 (2023).
Banerjee, A. Succinate produced by intestinal microbes promotes specification of tuft cells to suppress ileal inflammation. Gastroenterology 159, 2101–2115 (2020).
Sonnert, N. D., Rosen, C. E. & Ghazi, A. R. A host-microbiota interactome reveals extensive transkingdom connectivity. Nature 628, 171–179 (2024).
Zhang, S., Morgan, X. & Dogan, B. Mucosal metabolites fuel the growth and virulence of E. coli linked to Crohn’s disease. JCI Insight 7, e157013 (2022).
Gogokhia, L. et al. Donor composition and fiber promote strain engraftment in a randomized controlled trial of fecal microbiota transplant for ulcerative colitis. Med. https://doi.org/10.1016/j.medj.2025.100707 (2025).
Brodel, A. K., Charpenay, L. H. & Galtier, M. In situ targeted base editing of bacteria in the mouse gut. Nature 632, 877–884 (2024).
Russell, B. J., Brown, S. D. & Siguenza, N. Intestinal transgene delivery with native E. coli chassis allows persistent physiological changes. Cell 185, 3263–3277 (2022).
Galtier, M., Sordi, L. & Sivignon, A. Bacteriophages targeting adherent invasive Escherichia coli strains as a promising new treatment for Crohn’s disease. J. Crohns Colitis 11, 840–847 (2017).
Federici, S., Kredo-Russo, S. & Valdes-Mas, R. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185, 2879–2898 (2022).
Sharma, P. et al. The next decade of immune checkpoint therapy. Cancer Discov. 11, 838–857 (2021).
Blake, S. J., Wolf, Y., Boursi, B. & Lynn, D. J. Role of the microbiota in response to and recovery from cancer therapy. Nat. Rev. Immunol. 24, 308–325 (2024).
Matson, V. et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science 350, 1084–1089 (2015).
Derosa, L. et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 28, 315–324 (2022).
Fidelle, M. et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science 380, eabo2296 (2023).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Routy, B. et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Griffin, M. E. et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science 373, 1040–1046 (2021).
McCulloch, J. A. et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 28, 545–556 (2022).
Baruch, E. N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609 (2021).
Davar, D. et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science 371, 595–602 (2021).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16, 563–580 (2019).
Wang, Y., Jenq, R. R., Wargo, J. A. & Watowich, S. S. Microbiome influencers of checkpoint blockade–associated toxicity. J. Exp. Med. 220, 20220948 (2023).
Lo, B. C. Microbiota-dependent activation of CD4+ T cells induces CTLA-4 blockade–associated colitis via Fcγ receptors. Science 383, 62–70 (2024).
Hu, Z. I. Immune checkpoint inhibitors unleash pathogenic immune responses against the microbiota. Proc. Natl Acad. Sci. USA 119, 2200348119 (2022).
Wang, Y. et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 24, 1804–1808 (2018).
Wang, F., Yin, Q., Chen, L. & Davis, M. M. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc. Natl Acad. Sci. USA 115, 157–161 (2018).
Imdad, A., Pandit, N. G. & Zaman, M. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 4, CD012774 (2023).
Paramsothy, S., Nielsen, S. & Kamm, M. A. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology 156, 1440–1454 (2019).
Lima, S. F., Gogokhia, L. & Viladomiu, M. Transferable immunoglobulin A-coated odoribacter splanchnicus in responders to fecal microbiota transplantation for ulcerative colitis limits colonic inflammation. Gastroenterology 162, 166–178 (2022).
Haifer, C., Paramsothy, S. & Kaakoush, N. O. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 7, 141–151 (2022).
Zheng, W., Zhao, S. & Yin, Y. High-throughput, single-microbe genomics with strain resolution, applied to a human gut microbiome. Science 376, eabm1483 (2022).
Reichart, N. J., Steiger, A. K. & Fossen, E. M. Selection and enrichment of microbial species with an increased lignocellulolytic phenotype from a native soil microbiome by activity-based probing. ISME Commun. 3, 106 (2023).
Han, L., Pendleton, A. & Singh, A. Chemoproteomic profiling of substrate specificity in gut microbiota-associated bile salt hydrolases. Cell Chem. Biol. 32, 145–156 (2024).
Choi, Y. et al. Immune checkpoint blockade induces gut microbiota translocation that augments extraintestinal antitumor immunity. Sci. Immunol. 8, eabo2003 (2023).
Jia, D. et al. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell 187, 1651–1665 (2024).
He, Y. et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 33, 988–1000 (2021).
Mager, L. F. et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489 (2020).
Lam, K. C. et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 184, 5338–5356 (2021).
Galloway-Peña, J., Iliev, I. D. & McAllister, F. Fungi in cancer. Nat. Rev. Cancer 24, 295–298 (2024).
Dohlman, A. B. et al. The multi-kingdom cancer microbiome. Nat. Microbiol. https://doi.org/10.1038/s41564-025-02103-7 (2025).
Coutzac, C. et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 11, 2168 (2020).
Fluckiger, A. et al. Cross-reactivity between tumor MHC class I–restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 (2020).
Naghavian, R. et al. Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma. Nature 617, 807–817 (2023).
Kalaora, S. et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 592, 138–143 (2021).
Lancaster, S. M., Lee-McMullen, B. & Abbott, C. W. Global, distinctive, and personal changes in molecular and microbial profiles by specific fibers in humans. Cell Host Microbe 30, 848–862 (2022).
Wastyk, H. C., Fragiadakis, G. K. & Perelman, D. Gut-microbiota-targeted diets modulate human immune status. Cell 184, 4137–4153 (2021).
Delannoy-Bruno, O., Desai, C. & Raman, A. S. Evaluating microbiome-directed fibre snacks in gnotobiotic mice and humans. Nature 595, 91–95 (2021).
Mocanu, V., Zhang, Z. & Deehan, E. C. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat. Med. 27, 1272–1279 (2021).
Kedia, S., Virmani, S. & KV, S. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut 71, 2401–2413 (2022).
Costello, S. P., Day, A. & Yao, C. K. Faecal microbiota transplantation (FMT) with dietary therapy for acute severe ulcerative colitis. BMJ Case Rep. 13, e233135 (2020).
Killinger, B. J., Whidbey, C. & Sadler, N. C. Activity-based protein profiling identifies alternating activation of enzymes involved in the bifidobacterium shunt pathway or mucin degradation in the gut microbiome response to soluble dietary fiber. NPJ Biofilms Microbiomes 8, 60 (2022).
Park, H. B., Wei, Z. & Oh, J. Sulfamethoxazole drug stress upregulates antioxidant immunomodulatory metabolites in Escherichia coli. Nat. Microbiol. 5, 1319–1329 (2020).
Lima, S. F., Pires, S. & Rupert, A. The gut microbiome regulates the clinical efficacy of sulfasalazine therapy for IBD-associated spondyloarthritis. Cell Rep. Med. 5, 101431 (2024).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Link, V. M. et al. Differential peripheral immune signatures elicited by vegan versus ketogenic diets in humans. Nat. Med. 30, 560–572 (2024).
Siracusa, F. et al. Short-term dietary changes can result in mucosal and systemic immune depression. Nat. Immunol. https://doi.org/10.1038/s41590-023-01587-x (2023).
Jeong, M. & Collins, N. Nutritional modulation of antitumor immunity. Curr. Opin. Immunol. 87, 102422 (2024).
Collins, N. & Belkaid, Y. Control of immunity via nutritional interventions. Immunity 55, 210–223 (2022).
Spencer, C. N. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 374, 1632–1640 (2021).
Simpson, R. C. et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. 28, 2344–2352 (2022).
Collins, N. et al. The bone marrow protects and optimizes immunological memory during dietary restriction. Cell 178, 1088–1101 (2019).
Mao, Y. -Q. et al. The antitumour effects of caloric restriction are mediated by the gut microbiome. Nat. Metab. 5, 96–110 (2023).
Han, S. -J. et al. Microbiota configuration determines nutritional immune optimization. Proc. Natl Acad. Sci. USA 120, e2304905120 (2023).
Bolte, L. A. et al. Association of a Mediterranean diet with outcomes for patients treated with immune checkpoint blockade for advanced melanoma. JAMA Oncol. 9, 705–709 (2023).
Lussier, D. M. et al. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 16, 310 (2016).
Ferrere, G. et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight 6, e145207 (2021).
Rubio-Patiño, C. et al. Low-protein diet induces IRE1α-dependent anticancer immunosurveillance. Cell Metab. 27, 828–842 (2018).
Messaoudene, M. et al. A natural polyphenol exerts antitumor activity and circumvents Anti–PD-1 resistance through effects on the gut microbiota. Cancer Discov. 12, 1070–1087 (2022).
Huang, J. et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 71, 734–745 (2022).
Schulz, M. D. et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 514, 508–512 (2014).
Nakatsu, G., Andreeva, N., MacDonald, M. H. & Garrett, W. S. Interactions between diet and gut microbiota in cancer. Nat. Microbiol. 9, 1644–1654 (2024).
Wang, Z. et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25, 141–151 (2019).
Bader, J. E. et al. Obesity induces PD-1 on macrophages to suppress anti-tumour immunity. Nature https://doi.org/10.1038/s41586-024-07529-3 (2024).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Acknowledgements
We thank the following funding agencies for their support: US National Institutes of Health (NIH; R01DK113136, R01DK121977, R01AI178683, R01CA286920, R01AI163007, R01DK135816, AI172027, DK132244, AT013241, R01HD110118, R01HL169989, R01AI143842, R01AI123368, R01AI145989, U01AI095608, R01AI162936, R01CA274534, R37AI174468, R01DK126871, R01AI151599, R01AI095466, R01AR070116, R01AI182043, R00CA252443, R21AI178327, R01AI170832, R01AI170897), the Cancer Research Institute, the Sanders Family Foundation, the Rosanne H. Silbermann Foundation, Linda and Glenn Greenberg, the Allen Discovery Center Program, a Paul G. Allen Frontiers Group advised program of the Paul G. Allen Family Foundation, the Hartwell Foundation and Starr Cancer Consortium, the Leona M. and Harry B. Helmsley Charitable Trust, the Burroughs Wellcome Fund: Pathogenesis of Infectious Disease (PATH), Crohn’s and Colitis Foundation, Kenneth Rainin Foundation and the Canadian Institute for Advanced Research (CIFAR) Fungal Kingdom: Threats and Opportunities research program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Andreas Diefenbach and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Jamie D. K. Wilson, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iliev, I.D., Blander, J.M., Collins, N. et al. Microbiota-mediated mechanisms of mucosal immunity across the lifespan. Nat Immunol 26, 1645–1659 (2025). https://doi.org/10.1038/s41590-025-02281-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41590-025-02281-w