Fig. 1: Demographics of clinical trial participants and trial schedule.
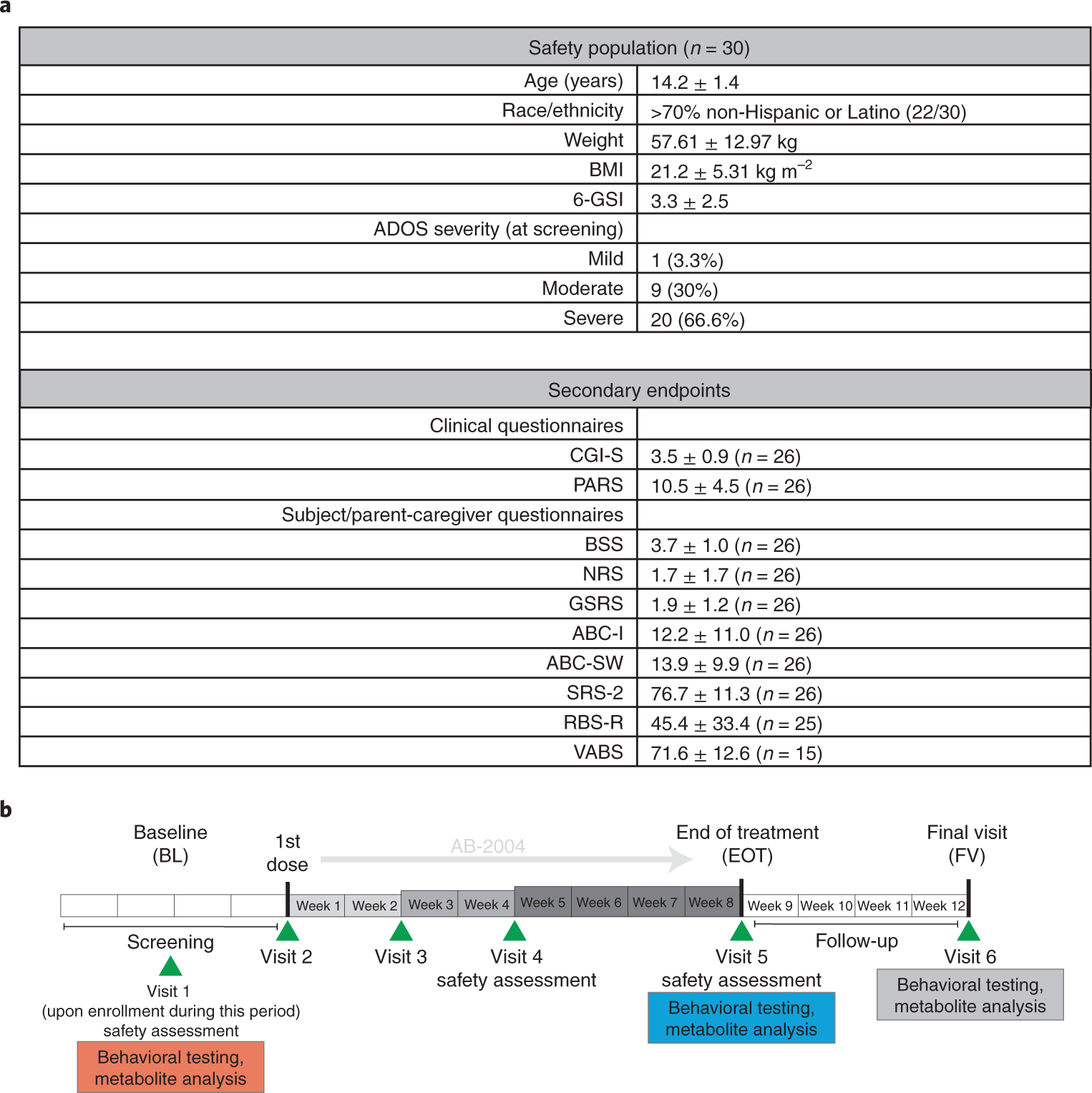
a, Trial demographics and metadata summary of participants. b, Phase 1 clinical trial schedule. Participants were screened during a 4-week run-in period, followed by dose escalation in weeks 0–2, weeks 2–4 and weeks 4–8, with a follow-up 4 weeks after trial. ABC-SW, Aberrant Behavior Checklist-Social Withdrawal; BMI, body mass index.
