Abstract
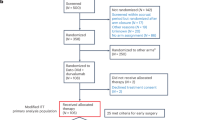
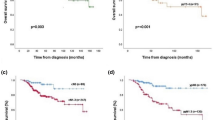
Among the goals of patient-centric care are the advancement of effective personalized treatment, while minimizing toxicity. The phase 2 I-SPY2.2 trial uses a neoadjuvant sequential therapy approach in breast cancer to further these goals, testing promising new agents while optimizing individual outcomes. Here we tested datopotamab–deruxtecan (Dato-DXd) in the I-SPY2.2 trial for patients with high-risk stage 2/3 breast cancer. I-SPY2.2 uses a sequential multiple assignment randomization trial design that includes three sequential blocks of biologically targeted neoadjuvant treatment: the experimental agent(s) (block A), a taxane-based regimen tailored to the tumor subtype (block B) and doxorubicin–cyclophosphamide (block C). Patients are randomized into arms consisting of different investigational block A treatments. Algorithms based on magnetic resonance imaging and core biopsy guide treatment redirection after each block, including the option of early surgical resection in patients predicted to have a high likelihood of pathological complete response, the primary endpoint. There are two primary efficacy analyses: after block A and across all blocks for the six prespecified breast cancer subtypes (defined by clinical hormone receptor/human epidermal growth factor receptor 2 (HER2) status and/or the response-predictive subtypes). We report results of 103 patients treated with Dato-DXd. While Dato-DXd did not meet the prespecified threshold for success (graduation) after block A in any subtype, the treatment strategy across all blocks graduated in the hormone receptor-negative HER2−Immune−DNA repair deficiency− subtype with an estimated pathological complete response rate of 41%. No new toxicities were observed, with stomatitis and ocular events occurring at low grades. Dato-DXd was particularly active in the hormone receptor-negative/HER2−Immune−DNA repair deficiency− signature, warranting further investigation, and was safe in other subtypes in patients who followed the treatment strategy. ClinicalTrials.gov registration: NCT01042379.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
De-identified subject level data and/or clinical specimens are made available to members of the research community upon approval of the I-SPY Data Access and Publications Committee. Details of the application and review process are available at https://www.quantumleaphealth.org/for-investigators/clinicians-proposal-submissions/. I-SPY aims to make complete patient-level clinical datasets available for public access within 6 months of publication, as the data is complex and requires extensive annotation to ensure its usability.
Code availability
The statistical code used in this clinical trial is available to other investigators for approved research purposes. Investigators interested in accessing the code complete an application at https://www.quantumleaphealth.org/for-investigators/clinicians-proposal-submissions/ and include a brief description of the intended use. Access to the code will be granted upon approval of the request, subject to compliance with ethical guidelines and applicable institutional and regulatory requirements.
References
Nanda, R. et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer. JAMA Oncol. 6, 676–684 (2020).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
I-SPY2 Trial Consortium. Association of event-free and distant recurrence–free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer. JAMA Oncol. 6, 1355–1362 (2020).
Li, W. et al. Abstract P4-02-10: MRI models by response predictive subtype for predicting pathologic complete response. Cancer Res. 83, P4-02-10 (2023).
Wolf, D. M. et al. Redefining breast cancer subtypes to guide treatment prioritization and maximize response: Predictive biomarkers across 10 cancer therapies. Cancer Cell 40, 609–623.e6 (2022).
Onishi, N. et al. Abstract P3-03-01: functional tumor volume at 3 and 6 week MRI as an indicator of patients with inferior outcome after neoadjuvant chemotherapy. Cancer Res. 82, P3-03-01 (2022).
Onishi, N. et al. Prospective performance of an MRI algorithm for early re-direction of breast cancer neoadjuvant treatment. In Proc. 32nd Annual Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine (International Society for Magnetic Resonance in Medicine, 2024).
Okajima, D. et al. Datopotamab deruxtecan (Dato-DXd), a novel TROP2-directed antibody–drug conjugate, demonstrates potent antitumor activity by efficient drug delivery to tumor cells. Mol. Cancer Ther. 20, 2329–2340 (2021).
Sakach, E., Sacks, R. & Kalinsky, K. Trop-2 as a therapeutic target in breast cancer. Cancers 14, 5936 (2022).
Bardia, A. et al. Datopotamab deruxtecan in advanced or metastatic HR+/HER2− and triple-negative breast cancer: results from the phase I TROPION-PanTumor01 study. J. Clin. Oncol. 42, 2281–2294 (2024).
Gadaleta-Caldarola, G. et al. Safety evaluation of datopotamab deruxtecan for triple-negative breast cancer: a meta-analysis. Cancer Treat. Res. Commun. 37, 100775 (2023).
Dent, R. A. et al. TROPION-Breast02: datopotamab deruxtecan for locally recurrent inoperable or metastatic triple-negative breast cancer. Future Oncol. 19, 2349–2359 (2023).
Bardia, A. et al. TROPION-Breast03: a randomized phase III global trial of datopotamab deruxtecan ± durvalumab in patients with triple-negative breast cancer and residual invasive disease at surgical resection after neoadjuvant therapy. Ther. Adv. Med. Oncol. 16, 17588359241248336 (2024).
Bardia, A. et al. TROPION-Breast01: datopotamab deruxtecan vs chemotherapy in pre-treated inoperable or metastatic HR+/HER2− breast cancer. Futur. Oncol. 20, 423–436 (2024).
Rugo, H. S. et al. Adaptive randomization of veliparib–carboplatin treatment in breast cancer. N. Engl. J. Med. 375, 23–34 (2016).
Shatsky, R. A. et al. Datopotamab–deruxtecan plus durvalumab in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial. Nat. Med. https://doi.org/10.1038/s41591-024-03267-1 (2024).
Lavori, P. W. & Dawson, R. Introduction to dynamic treatment strategies and sequential multiple assignment randomization. Clin. Trials 11, 393–399 (2014).
Li, W. et al. Predicting breast cancer response to neoadjuvant treatment using multi-feature MRI: results from the I-SPY 2 TRIAL. NPJ Breast Cancer 6, 63 (2020).
Symmans, W. F. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 25, 4414–4422 (2007).
Yau, C. et al. Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol. 23, 149–160 (2022).
Common terminology criteria for adverse events (CTCAE) protocol development. CTEP National Cancer Institute https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (2021).
Basch, E. et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J. Natl Cancer Inst. 106, dju244 (2014).
Jacob, S. et al. Use of PROMIS to capture patient reported outcomes over time for patients on I-SPY2. J. Clin. Oncol. 41, 611 (2023).
Pearman, T. P., Beaumont, J. L., Mroczek, D., O’Connor, M. & Cella, D. Validity and usefulness of a single-item measure of patient-reported bother from side effects of cancer therapy. Cancer 124, 991–997 (2018).
Oken, M. M. et al. Toxicity and response criteria of the eastern-cooperative-oncology-group. Am. J. Clin. Oncol. 5, 649–655 (1982).
Cardoso, F. et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N. Engl. J. Med. 375, 717–729 (2016).
Beltran, P. J. et al. Ganitumab (AMG 479) inhibits IGF-II–dependent ovarian cancer growth and potentiates platinum-based chemotherapy. Clin. Cancer Res. 20, 2947–2958 (2014).
Pusztai, L. et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial. Cancer Cell 39, 989–998.e5 (2021).
Piccart, M. et al. 70-Gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 22, 476–488 (2021).
Brahmer, J. R. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 9, e002435 (2021).
Haanen, J. et al. Management of toxicities from immunotherapy: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33, 1217–1238 (2022).
Acknowledgements
Research reported in this paper was supported by the NCI of the National Institutes of Health (NIH) under award numbers P01CA210961 and U01CA225427 (L.J.E., N.H.). We acknowledge the generous support of the study sponsors and operations management, Quantum Leap Healthcare Collaborative (QLHC, 2013 to present) and the Foundation for the NIH (2010 to 2012; to L.J.E.). We sincerely appreciate the ongoing support for the I-SPY2.2 Trial from the Safeway Foundation, the William K. Bowes, Jr. Foundation, Give Breast Cancer the Boot and QLHC (all to L.J.E.) and the Breast Cancer Research Foundation (to L.J.E. and L.J.v.V.). We thank all the patients who volunteered to participate in I-SPY2. AstraZeneca provided funds and study drugs (datopotamab–deruxtecan and durvalumab). With the exception of QLHC, the funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank D. Wolf and J. Gibbs and the I-SPY patient advocates for all their important contributions to this work.
Author information
Authors and Affiliations
Contributions
Arm chaperones: J.L.M. and K.K. Leadership: C.Y., H.S.R., R.N., B.M., J.P., P.P., R.A.S., A.D., D.Y., L.J.v.V., N.M.H. and L.J.E. Study design: C.Y., M.D., B. Tsiatis, A.D., D.Y., L.J.v.V., N.M.H. and L.J.E. Formal analysis: C.Y. and P.N. Enrolled patients: J.L.M., K.K., H.S.R., R.N., A.J.C., A.M.W., M.A., M.R., D.L.H., A.Z., A.S.C., H.B., A.D.E., E.S.-R., J.C.B., C.N., C.V., C.O., K.S.A., K.M.K., C.I., J.T., E.T.R.T., B.T., A.T., A.S., R.B., C.E., K.Y., C.S., T.S., L.P., M.S.T., A.O., R.A.S., A.D., D.Y. and L.J.E. Laboratory studies: L.B.-S., G.L.H. and L.J.v.V. Imaging/pathology: W.L., N.O., W.F.S. and N.M.H. Data management: A.L.A. and P.B. Administration: G.L.H. and J.B.M. First draft: J.L.M., K.K., C.Y., P.N., J.B.M. and L.J.E. Edit and review: J.L.M., K.K., C.Y., H.S.R., R.N., M.D., B. Tsiatis, A.J.C., A.M.W., M.A., M.R., D.L.H., A.Z., A.S.C., H.B., A.D.E., E.S.-R., J.C.B., C.N., C.V., C.O., K.S.A., K.M.K., C.I., J.T., E.T.R.T., B. Thomas, A.T., A.S., R.B., C.E., K.Y., C.S., T.S., L.P., M.S.T., A.O., W.L., N.O., A.L.A., P.B., P.N., L.B.-S., G.L.H., J.B.M., B.M., W.F.S., E.P., C.B., J.P., P.P., R.A.S., A.D., D.Y., L.J.v.V., N.M.H. and L.J.E.
Corresponding author
Ethics declarations
Competing interests
J.L.M. reports institutional research funding from AstraZeneca, Seagen, Sermonix and Olema and advisory and consulting roles with Pfizer, Seagen, Sermonix, Novartis, Stemline, AstraZeneca, Olema, GlaxoSmithKline and GE Healthcare. C.Y. reports institutional research grant from NCI/NIH; salary support and travel reimbursement from QLHC; a United States patent titled ‘Breast cancer response prediction subtypes’ (no. 18/174,491); and University of California Inventor Share. H.S.R. reports institutional research support from AstraZeneca, Daiichi Sankyo, Inc., F. Hoffmann–La Roche AG/Genentech, Inc., Gilead Sciences, Inc., Lilly; Merck and Co., Novartis Pharmaceuticals Corporation, Pfizer, Stemline Therapeutics, OBI Pharma, Ambrx, Greenwich Pharma; and advisory and consulting roles with Chugai, Puma, Sanofi, Napo and Mylan. R.N. reports research funding from Arvinas, AstraZeneca, BMS, Corcept Therapeutics, Genentech/Roche, Gilead, GSK, Merck, Novartis, OBI Pharma, OncoSec, Pfizer, Relay, Seattle Genetics, Sun Pharma and Taiho and advisory roles with AstraZeneca, BeyondSpring, Daiichi Sankyo, Exact Sciences, Fujifilm, GE, Gilead, Guardant Health, Infinity, iTeos, Merck, Moderna, Novartis, OBI, OncoSec, Pfizer, Sanofi, Seagen and Stemline. M.D. reports research grants from NIH/NCI and NIH/NIA, and contracts from PCORI. A.J.C. reports institutional research funding from Merck, Amgen, Puma, Seagen, Pfizer and Olema and advisory roles with AstraZeneca and Genentech. A.Z. reports institutional research funding from Merck, honoraria for Medscape and participation on Pfizer Advisory Board. A.S.C. reports institutional research funding from Novartis and Lilly. A.D.E. reports support from Scorpion, Infinity and Deciphera. E.S.-R. reports grants from V Foundation, NIH, Susan G. Komen; institutional research funding from GSK, Seagen, Pfizer, Lilly; consulting and honoraria from Novartis, Merck, Seagen, AstraZeneca, Lilly; Cancer Awareness Network Board member and support from ASCO and NCCN. J.C.B. reports institutional research funding from Eli Lilly and SymBioSis, participation on the Data Safety Monitoring Committee of Cairn Surgical and honoraria from PER, PeerView, OncLive, EndoMag and UpToDate. C.V. reports institutional research funding from Pfizer, Seagen, H3 Biomedicine/Eisai, AstraZeneca, CytomX; research funding to previous institution from Genentech, Roche, Pfizer, Incyte, Pharmacyclics, Novartis, TRACON Pharmaceuticals, Innocrin Pharmaceuticals, Zymeworks and H3 Biomedicine; advisory and consulting roles with Guidepoint, Novartis, Seagen, Daiichi Sankyo, AstraZeneca and Cardinal Health; unpaid consulting with Genentech; and participation in non-CME activity with Gilead, AstraZeneca. C.O. reports consulting fees from AstraZeneca, Guardant Health and Jazz Pharmaceuticals. K.S.A. reports institutional research funding from AstraZeneca, Daiichi Sankyo, Seattle Genetics and QLHC; Independent Data and Safety Monitoring committee at Seattle Genetics. K.M.K. reports advisory and consultant roles for Eli Lilly, Pfizer, Novartis, AstraZeneca, Daiichi Sankyo, Puma, 4D Pharma, OncoSec, Immunomedics, Merck, Seagen, Mersana, Menarini Silicon Biosystems, Myovant, Takeda, Biotheranostics, Regor, Gilead, Prelude Therapeutics, RayzeBio, eFFECTOR Therapeutics and Cullinan Oncology; and reports institutional research funding from Genentech/Roche, Novartis, Eli Lilly, AstraZeneca, Daiichi Sankyo and Ascentage. C.I. reports institutional research funding from Tesaro/GSK, Seattle Genetics, Pfizer, AstraZeneca, BMS, Genentech, Novartis and Regeneron; consultancy roles with AstraZeneca, Genentech, Gilead, ION, Merck, Medscape, MJH Holdings, Novartis, Pfizer, Puma and Seagen; and royalties from Wolters Kluwer (UptoDate) and McGraw Hill (Goodman and Gillman). J.T. serves as institutional principal investigator for clinical trial with Intuitive Surgical; editor lead for ABS, CGSO, SCORE, Breast Education Committee Track Leader, ASCO SESAP 19 and Breast Co-Chair, ACS. A.T. owns stock at Johnson and Johnsons, Gilead, Bristol Myers Squibb, reports participation on Pfizer Advisory Board: AstraZeneca and reports institutional research funding from Merck and Sanofi and royalties from UptoDate. R.B. reports a consultancy role at Genentech and stock ownership at Cerus Corp. K.Y. received research support unrelated to this work and paid to the institution from Pfizer, Gilead, Seagen, Dantari Pharmaceuticals, Treadwell Therapeutics, and Relay Therapeutics; support from American Cancer Society IRG grant no. IRG-19-230-48-IRG, UC San Diego Moores Cancer Center, Specialized Cancer Center support grant NIH/NCI P30CA023100, Curebound Discovery Award (2023, 2024). T.S. reports honoraria from Hologic. L.P. reports institutional research funding from Susan Komen Foundation, Breast Cancer Research Foundation, NCI, Pfizer, AstraZeneca, Menarini/Stemline, Bristol Myers Squibb, Merck and Co.; consulting fees from AstraZeneca, Merck, Novartis, Genentech, Natera, Personalis, Exact Sciences and Stemline/Menarini; patent titled ‘Method of measuring residual cancer and predicting patient survival’ (no. 7711494); and Data and Safety Monitoring Board member of the DYNASTY Breast02, OPTIMA and PARTNER trials. M.S.T. reports institutional research funding from Lilly, Gilead Sciences, Phoenix Molecular Designs, AstraZeneca, Regeneron, Merck and Novartis. A.L.A., P.B. and P.N. are employees of QLHC. G.L.H. reports institutional research grant from NIH (1R01CA255442). W.F.S. reports shares of IONIS Pharmaceuticals and Eiger Biopharmaceuticals, received consulting fees from AstraZeneca, is a cofounder with equity in Delphi Diagnostics and issued patents for (1) a method to calculate residual cancer burden and (2) genomic signature to measure sensitivity to endocrine therapy. J.P. reports honoraria from Methods in Clinical Research—faculty SCION workshop; support from ASCO and advocate scholarship; AACR—SSP program; VIVLI, U Wisc SPORE—EAB, QuantumLEAD—COVID DSMB, PCORI—reviewer and I-SPY advocate lead. P.P. reports institutional research funding from Genentech/Roche, Fabre-Kramer, Advanced Cancer Therapeutics, Caris Centers of Excellence, Pfizer, Pieris Pharmaceuticals, Cascadian Therapeutics, Bolt, Byondis, Seagen, Orum Therapeutics and Carisma Therapeutics; consulting fees from Personalized Cancer Therapy, OncoPlex Diagnostics, Immunonet BioSciences, Pfizer, HERON, Puma Biotechnology, Sirtex, CARIS Life sciences, Juniper, Bolt Biotherapeutics and AbbVie; honoraria from DAVA Oncology, OncLive/MJH Life Sciences, Frontiers—publisher, SABCS and ASCO; Speakers’ Bureau: Genentech/Roche (past); United States patent no. 8486413, United States patent no. 8501417, United States patent no. 9023362, United States patent no. 9745377; uncompensated roles with Pfizer, Seagen and Jazz. R.A.S. reports institutional research funding from OBI Pharma, QLHC, AstraZeneca and Gilead, serves on AstraZeneca and Stemline Advisory Boards and Gilead Speaker’s Bureau and reports consultancy role with QLHC. A.D. reports institutional research funding from Novartis, Pfizer, Genentech and NeoGenomics; Program Chair, Scientific Advisory Committee, ASCO. D.Y. reports research funding from NIH/NCI P30 CA 077598, P01 CA234228-01 and R01CA251600, consulting fees from Martell Diagnostics, and honoraria and travel for speaking at the ‘International Breast Cancer Conference.’ L.J.v.V. is a founding advisor and shareholder of Exai Bio and is a part-time employee and owns stock in Agendia. N.M.H. reports institutional research funding from NIH. L.J.E. reports funding from Merck and Co., participation on an advisory board for Blue Cross Blue Shield and personal fees from UpToDate and is an unpaid board member of QLHC. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Barbara Pistilli and Sze-Huey Tan for their contribution to the peer review of this work. Primary Handling Editor: Ulrike Harjes, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures and tables.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khoury, K., Meisel, J.L., Yau, C. et al. Datopotamab–deruxtecan in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial. Nat Med 30, 3728–3736 (2024). https://doi.org/10.1038/s41591-024-03266-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-024-03266-2
This article is cited by
-
Antibody-drug conjugates in breast cancer: current evidence and future directions
Experimental Hematology & Oncology (2025)
-
Circulating tumor DNA refines risk stratification of neoadjuvant therapy-resistant breast tumors
Nature Communications (2025)
-
Moving toward response-adapted trials in oncology
Nature Medicine (2024)