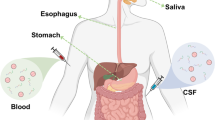
Abstract
Cerebral accumulation of alpha-synuclein (αSyn) aggregates is the hallmark event in a group of neurodegenerative diseases—collectively called synucleinopathies—which include Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy. Currently, these are diagnosed by their clinical symptoms and definitively confirmed postmortem by the presence of αSyn deposits in the brain. Here, we summarize the drawbacks of the current clinical definition of synucleinopathies and outline the rationale for moving toward an earlier, biology-anchored definition of these disorders, with or without the presence of clinical symptoms. We underscore the utility of the αSyn seed amplification assay to detect aggregated αSyn in living patients and to differentiate between neuronal or glial αSyn pathology. We anticipate that a biological definition of synucleinopathies, if well-integrated with the current clinical classifications, will enable further understanding of the disease pathogenesis and contribute to the development of effective, disease-modifying therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bras, I. C. et al. Synucleinopathies: where we are and where we need to go. J. Neurochem. 153, 433–454 (2020).
Goedert, M., Jakes, R. & Spillantini, M. G. The synucleinopathies: twenty years on. J. Parkinsons Dis. 7, S53–S71 (2017).
Poewe, W. et al. Diagnosis and management of Parkinson’s disease dementia. Int. J. Clin. Pract. 62, 1581–1587 (2008).
Rocca, W. A. The future burden of Parkinson’s disease. Mov. Disord. 33, 8–9 (2018).
Desai, U. et al. Epidemiology and economic burden of Lewy body dementia in the United States. Curr. Med. Res. Opin. 38, 1177–1188 (2022).
Armstrong, M. J. & Okun, M. S. Diagnosis and treatment of Parkinson disease: a review. JAMA 323, 548–560 (2020).
Rizzo, G. et al. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86, 566–576 (2016).
Adler, C. H. et al. Clinical diagnostic accuracy of early/advanced parkinson disease: an updated clinicopathologic study. Neurol. Clin. Pract. 11, e414–e421 (2021).
Wenning, G. K., Krismer, F. & Poewe, W. New insights into atypical parkinsonism. Curr. Opin. Neurol. 24, 331–338 (2011).
Kon, T., Tomiyama, M. & Wakabayashi, K. Neuropathology of Lewy body disease: clinicopathological crosstalk between typical and atypical cases. Neuropathology 40, 30–39 (2020).
Twohig, D. & Nielsen, H. M. Alpha-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 14, 23 (2019).
Chung, E. J. et al. Clinical features of Alzheimer disease with and without lewy bodies. JAMA Neurol. 72, 789–796 (2015).
Palmqvist, S. et al. Cognitive effects of Lewy body pathology in clinically unimpaired individuals. Nat. Med. 29, 1971–1978 (2023).
Quadalti, C. et al. Clinical effects of Lewy body pathology in cognitively impaired individuals. Nat. Med. 29, 1964–1970 (2023).
Hampel, H. et al. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 17, 580–589 (2021).
Jack, C. R. Jr. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562 (2018).
Jack, C. R. Jr. et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87, 539–547 (2016).
Hoglinger, G. U. et al. A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria. Lancet Neurol. 23, 191–204 (2024).
Simuni, T. et al. A biological definition of neuronal alpha-synuclein disease: towards an integrated staging system for research. Lancet Neurol. 23, 178–190 (2024).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89, 88–100 (2017).
Gilman, S. et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71, 670–676 (2008).
Wenning, G. K. et al. The movement disorder society criteria for the diagnosis of multiple system atrophy. Mov. Disord. 37, 1131–1148 (2022).
Greffard, S. et al. Motor score of the unified Parkinson disease rating scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch. Neurol. 63, 584–588 (2006).
Neikrug, A. B. et al. Parkinson’s disease and REM sleep behavior disorder result in increased non-motor symptoms. Sleep. Med. 15, 959–966 (2014).
Mahlknecht, P., Seppi, K. & Poewe, W. The concept of prodromal Parkinson’s disease. J. Parkinsons Dis. 5, 681–697 (2015).
Berg, D. et al. Prodromal Parkinson disease subtypes — key to understanding heterogeneity. Nat. Rev. Neurol. 17, 349–361 (2021).
Berg, D. et al. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 30, 1600–1611 (2015).
van de Beek, M. et al. Prodromal dementia with Lewy bodies: clinical characterization and predictors of progression. Mov. Disord. 35, 859–867 (2020).
Wyman-Chick, K. A. et al. Prodromal dementia with Lewy bodies: evolution of symptoms and predictors of dementia onset. J. Geriatr. Psychiatry Neurol. 35, 527–534 (2021).
McKeith, I. G. et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 94, 743–755 (2020).
Fereshtehnejad, S. M. et al. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study. Brain 142, 2051–2067 (2019).
Williams, D. R. & Litvan, I. Parkinsonian syndromes. Continuum19, 1189–1212 (2013).
Arvanitakis, Z., Shah, R. C. & Bennett, D. A. Diagnosis and management of dementia: review. JAMA 322, 1589–1599 (2019).
Park, K. W. et al. Dementia with Lewy bodies versus Alzheimer’s disease and Parkinson’s disease dementia: a comparison of cognitive profiles. J. Clin. Neurol. 7, 19–24 (2011).
Hohl, U., Tiraboschi, P., Hansen, L. A., Thal, L. J. & Corey-Bloom, J. Diagnostic accuracy of dementia with Lewy bodies. Arch. Neurol. 57, 347–351 (2000).
Tiraboschi, P. et al. What best differentiates Lewy body from Alzheimer’s disease in early-stage dementia? Brain 129, 729–735 (2006).
McFarthing, K., Rafaloff, G., Baptista, M. A. S., Wyse, R. K. & Stott, S. R. W. Parkinson’s disease drug therapies in the clinical trial pipeline: 2021 update. J. Parkinsons Dis. 11, 891–903 (2021).
Salvado, G. et al. Disease staging of Alzheimer’s disease using a CSF-based biomarker model. Nat. Aging 4, 694–708 (2024).
Reiman, E. M. Drug trial for Alzheimer’s disease is a game changer. Nature 615, 42–43 (2023).
Huang, L. K., Kuan, Y. C., Lin, H. W. & Hu, C. J. Clinical trials of new drugs for Alzheimer disease: a 2020–2023 update. J. Biomed. Sci. 30, 83 (2023).
Darweesh, S. K. L., Sampaio, C. & Bloem, B. R. Has the time come to redefine Parkinson’s disease? Lancet Neurol. 23, 130–133 (2024).
Dickson, D. W. Neuropathology of Parkinson disease. Parkinsonism Relat. Disord. 46, S30–S33 (2018).
Miller, K. M., Mercado, N. M. & Sortwell, C. E. Synucleinopathy-associated pathogenesis in Parkinson’s disease and the potential for brain-derived neurotrophic factor. NPJ Parkinsons Dis. 7, 35 (2021).
Parnetti, L. et al. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 18, 573–586 (2019).
Berman, S. B. & Miller-Patterson, C. PD and DLB: brain imaging in Parkinson’s disease and dementia with Lewy bodies. Prog. Mol. Biol. Transl. Sci. 165, 167–185 (2019).
Chen-Plotkin, A. S. et al. Finding useful biomarkers for Parkinson’s disease. Sci. Transl. Med. 10, eaam6003 (2018).
Korat, S. et al. Alpha-synuclein PET tracer development—an overview about current efforts. Pharmaceuticals 14, 847 (2021).
Alzghool, O. M., van Dongen, G., van de Giessen, E., Schoonmade, L. & Beaino, W. Alpha-synuclein radiotracer development and in vivo imaging: recent advancements and new perspectives. Mov. Disord. 37, 936–948 (2022).
Eusebi, P. et al. Diagnostic utility of cerebrospinal fluid alpha-synuclein in Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 32, 1389–1400 (2017).
Donadio, V. et al. Skin nerve alpha-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology 82, 1362–1369 (2014).
Simonsen, A. H. et al. The utility of alpha-synuclein as biofluid marker in neurodegenerative diseases: a systematic review of the literature. Biomark. Med. 10, 19–34 (2015).
Atik, A., Stewart, T. & Zhang, J. Alpha-synuclein as a biomarker for parkinson’s disease. Brain Pathol. 26, 410–418 (2016).
Gao, L. et al. Cerebrospinal fluid alpha-synuclein as a biomarker for Parkinson’s disease diagnosis: a systematic review and meta-analysis. Int. J. Neurosci. 125, 645–654 (2015).
Ganguly, U. et al. Alpha-synuclein as a biomarker of Parkinson’s disease: good, but not good enough. Front. Aging Neurosci. 13, 702639 (2021).
Hall, S. et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 69, 1445–1452 (2012).
Soto, C. Alpha-synuclein seed amplification technology for Parkinson’s disease and related synucleinopathies. Trends Biotechnol. 42, 829–841 (2024).
Concha-Marambio, L., Pritzkow, S., Shahnawaz, M., Farris, C. M. & Soto, C. Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid. Nat. Protoc. 18, 1179–1196 (2023).
Shahnawaz, M. et al. Development of a biochemical diagnosis of parkinson disease by detection of alpha-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 74, 163–172 (2017).
Fairfoul, G. et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 3, 812–818 (2016).
Siderowf, A. et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using alpha-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 22, 407–417 (2023).
Concha-Marambio, L. et al. Accurate detection of alpha-synuclein seeds in cerebrospinal fluid from isolated rapid eye movement sleep behavior disorder and patients with Parkinson’s disease in the DeNovo Parkinson (DeNoPa) cohort. Mov. Disord. 38, 567–578 (2023).
Russo, M. J. et al. High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol. Commun. 9, 179 (2021).
Concha-Marambio, L. et al. Seed amplification assay to diagnose early Parkinson’s and predict dopaminergic deficit progression. Mov. Disord. 36, 2444–2446 (2021).
Singer, W. et al. Alpha-synuclein oligomers and neurofilament light chain predict phenoconversion of pure autonomic failure. Ann. Neurol. 89, 1212–1220 (2021).
Shahnawaz, M. et al. Discriminating alpha-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 578, 273–277 (2020).
Singer, W. et al. Alpha-synuclein oligomers and neurofilament light chain in spinal fluid differentiate multiple system atrophy from lewy body synucleinopathies. Ann. Neurol. 88, 503–512 (2020).
Kang, U. J. et al. Comparative study of cerebrospinal fluid alpha-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov. Disord. 34, 536–544 (2019).
Nakagaki, T., Nishida, N. & Satoh, K. Development of alpha-synuclein real-time quaking-induced conversion as a diagnostic method for alpha-synucleinopathies. Front. Aging Neurosci. 13, 703984 (2021).
Rossi, M. et al. Diagnostic value of the CSF alpha-synuclein real-time quaking-induced conversion assay at the prodromal MCI stage of dementia with Lewy bodies. Neurology 97, e930–e940 (2021).
Mammana, A. et al. RT-QuIC detection of pathological alpha-synuclein in skin punches of patients with Lewy body disease. Mov. Disord. 36, 2173–2177 (2021).
Han, J. Y., Shin, C. & Choi, Y. P. Preclinical detection of alpha-synuclein seeding activity in the colon of a transgenic mouse model of synucleinopathy by RT-QuIC. Viruses 13, 759 (2021).
Bargar, C. et al. Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 9, 62 (2021).
Manne, S. et al. Alpha-synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov. Disord. 35, 268–278 (2020).
Wang, Z. et al. Skin alpha-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol. 78, 1–11 (2020).
Manne, S. et al. Blinded RT-QuIC analysis of alpha-synuclein biomarker in skin tissue from Parkinson’s disease patients. Mov. Disord. 35, 2230–2239 (2020).
Park, S. J. et al. Establishment of method for the determination of aggregated alpha-synuclein in DLB patient using RT-QuIC assay. Protein Pept. Lett. 28, 115–120 (2020).
Rossi, M. et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 140, 49–62 (2020).
Han, J. Y., Jang, H. S., Green, A. J. E. & Choi, Y. P. RT-QuIC-based detection of alpha-synuclein seeding activity in brains of dementia with Lewy body patients and of a transgenic mouse model of synucleinopathy. Prion 14, 88–94 (2020).
Bongianni, M. et al. Alpha-synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann. Clin. Transl. Neurol. 6, 2120–2126 (2019).
Groveman, B. R. et al. Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta Neuropathol. Commun. 6, 7 (2018).
Iranzo, A. et al. Detection of alpha-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. 20, 203–212 (2021).
Okuzumi, A. et al. Propagative alpha-synuclein seeds as serum biomarkers for synucleinopathies. Nat. Med. 29, 1448–1455 (2023).
Iranzo, A. et al. Misfolded alpha-synuclein assessment in the skin and CSF by RT-QuIC in isolated REM sleep behavior disorder. Neurology 100, e1944–e1954 (2023).
Kuzkina, A. et al. Dermal real-time quaking-induced conversion is a sensitive marker to confirm isolated rapid eye movement sleep behavior disorder as an early alpha-synucleinopathy. Mov. Disord. 38, 1077–1082 (2023).
Vivacqua, G. et al. Salivary alpha-synuclein RT-QuIC correlates with disease severity in de novo Parkinson’s disease. Mov. Disord. 38, 153–155 (2023).
Bongianni, M. et al. Olfactory swab sampling optimization for alpha-synuclein aggregate detection in patients with Parkinson’s disease. Transl. Neurodegener. 11, 37 (2022).
Luan, M. et al. Diagnostic value of salivary real-time quaking-induced conversion in Parkinson’s disease and multiple system atrophy. Mov. Disord. 37, 1059–1063 (2022).
Fernandes Gomes, B. et al. Alpha-synuclein seed amplification assay as a diagnostic tool for parkinsonian disorders. Parkinsonism Relat. Disord. 117, 105807 (2023).
Arnold, M. R. et al. Alpha-synuclein seed amplification in CSF and brain from patients with different brain distributions of pathological alpha-synuclein in the context of co-pathology and non-LBD diagnoses. Ann. Neurol. 92, 650–662 (2022).
Chahine, L. M. et al. Central and peripheral alpha-synuclein in Parkinson disease detected by seed amplification assay. Ann. Clin. Transl. Neurol. 10, 696–705 (2023).
Kluge, A. et al. Detection of neuron-derived pathological alpha-synuclein in blood. Brain 145, 3058–3071 (2022).
Koga, S., Sekiya, H., Kondru, N., Ross, O. A. & Dickson, D. W. Neuropathology and molecular diagnosis of synucleinopathies. Mol. Neurodegener. 16, 83 (2021).
Pagano, G., Niccolini, F. & Politis, M. Imaging in Parkinson’s disease. Clin. Med. 16, 371–375 (2016).
Bega, D. et al. Clinical utility of DaTscan in patients with suspected Parkinsonian syndrome: a systematic review and meta-analysis. NPJ Parkinsons Dis. 7, 43 (2021).
Chahine, L. M. et al. Dopamine transporter imaging predicts clinically-defined alpha-synucleinopathy in REM sleep behavior disorder. Ann. Clin. Transl. Neurol. 8, 201–212 (2021).
Siderowf, A. et al. Clinical and imaging progression in the PARS Cohort: long-term follow-up. Mov. Disord. 35, 1550–1557 (2020).
De Micco, R., Russo, A. & Tessitore, A. Structural MRI in idiopathic Parkinson’s disease. Int Rev. Neurobiol. 141, 405–438 (2018).
Catalan, M. et al. (123)I-metaiodobenzylguanidine myocardial scintigraphy in discriminating degenerative parkinsonisms. Mov. Disord. Clin. Pract. 8, 717–724 (2021).
Snow, B. J. Fluorodopa PET scanning in Parkinson’s disease. Adv. Neurol. 69, 449–457 (1996).
Meyer, P. T., Frings, L., Rucker, G. & Hellwig, S. (18)F-FDG PET in parkinsonism: differential diagnosis and evaluation of cognitive impairment. J. Nucl. Med. 58, 1888–1898 (2017).
Schneider, S. A. & Alcalay, R. N. Neuropathology of genetic synucleinopathies with parkinsonism: review of the literature. Mov. Disord. 32, 1504–1523 (2017).
McKeith, I. G. Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. Neurol. Clin. 18, 865–902 (2000).
Jellinger, K. A. & Korczyn, A. D. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med 16, 34 (2018).
Weintraub, D. What’s in a name? The time has come to unify Parkinson’s disease and dementia with Lewy bodies. Mov. Disord. 38, 1977–1981 (2023).
Yang, Y. et al. Structures of alpha-synuclein filaments from human brains with Lewy pathology. Nature 610, 791–795 (2022).
Valera, E. & Masliah, E. The neuropathology of multiple system atrophy and its therapeutic implications. Auton. Neurosci. 211, 1–6 (2018).
Ma, Y. et al. Sensitivity and specificity of a seed amplification assay for diagnosis of multiple system atrophy: a multicentre cohort study. Lancet Neurol. 23, 1225–1237 (2024).
Xue, C., Lin, T. Y., Chang, D. & Guo, Z. Thioflavin T as an amyloid dye: fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 4, 160696 (2017).
Brück, D. et al. Glia and alpha-synuclein in neurodegeneration: a complex interaction. Neurobiol. Dis. 85, 262–274 (2016).
Liguori, R. et al. A comparative blind study between skin biopsy and seed amplification assay to disclose pathological alpha-synuclein in RBD. NPJ Parkinsons Dis. 9, 34 (2023).
Donadio, V. et al. Skin biopsy may help to distinguish multiple system atrophy-parkinsonism from Parkinson’s disease with orthostatic hypotension. Mov. Disord. 35, 1649–1657 (2020).
Funayama, M., Nishioka, K., Li, Y. & Hattori, N. Molecular genetics of Parkinson’s disease: contributions and global trends. J. Hum. Genet 68, 125–130 (2023).
Day, J. O. & Mullin, S. The genetics of Parkinson’s disease and implications for clinical practice. Genes 12, 1006 (2021).
Schneider, S. A., Hizli, B. & Alcalay, R. N. Emerging targeted therapeutics for genetic subtypes of parkinsonism. Neurotherapeutics 17, 1378–1392 (2020).
Soto, C. & Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21, 1332–1340 (2018).
Uchikado, H., Lin, W. L., DeLucia, M. W. & Dickson, D. W. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J. Neuropathol. Exp. Neurol. 65, 685–697 (2006).
Bellomo, G. et al. Investigating alpha-synuclein co-pathology in Alzheimer’s disease by means of cerebrospinal fluid alpha-synuclein seed amplification assay. Alzheimers Dement. 20, 2444–2452 (2024).
Irwin, D. J. & Hurtig, H. I. The contribution of tau, amyloid-beta and alpha-synuclein pathology to dementia in Lewy body disorders. J. Alzheimers Dis. Parkinsonism 8, 444 (2018).
Salvadores, N., Shahnawaz, M., Scarpini, E., Tagliavini, F. & Soto, C. Detection of misfolded abeta oligomers for sensitive biochemical diagnosis of Alzheimer’s disease. Cell Rep. 7, 261–268 (2014).
kraus, A. et al. Seeding selectivity and ultrasensitive detection of tau aggregate conformers of Alzheimer disease. Acta Neuropathol. 137, 585–598 (2019).
Scialo, C. et al. TDP-43 real-time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun. 2, fcaa142 (2020).
Ingelsson, M. Alpha-synuclein oligomers-neurotoxic molecules in Parkinson’s disease and other Lewy body disorders. Front Neurosci. 10, 408 (2016).
Samudra, N. et al. Clinicopathological correlation of cerebrospinal fluid alpha-synuclein seed amplification assay in a behavioral neurology autopsy cohort. Alzheimers Dement. 20, 3334–3341 (2024).
Goedert, M., Masuda-Suzukake, M. & Falcon, B. Like prions: the propagation of aggregated tau and alpha-synuclein in neurodegeneration. Brain 140, 266–278 (2017).
Grossauer, A. et al. Alpha-synuclein seed amplification assays in the diagnosis of synucleinopathies using cerebrospinal fluid-a systematic review and meta-analysis. Mov. Disord. Clin. Pract. 10, 737–747 (2023).
Food and Drug Administration. Drug development tool letter of support DDT-MBQ-000157; https://www.fda.gov/media/181368/download (19 August 2024).
Acknowledgements
C.S. received funding from NIH grants R01AG055053, U24AG079685 and R01AG079685, as well as grants from the Michael J. Fox Foundation for Parkinson’s Research (MJFF). B.M. is a member of the executive steering committee of the Parkinson Progression Marker Initiative of the MJFF and has received research funding from MJFF and Aligning Science Across Parkinson’s disease (ASAP, CRN). O.H. is supported by the European Research Council (ADG-101096455), the Alzheimer’s Association (ZEN24-1069572, SG-23-1061717), the GHR Foundation, the Swedish Research Council (2022-00775), ERA PerMed (ERAPERMED2021-184), the Knut and Alice Wallenberg foundation (2022-0231), Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University, the Swedish Alzheimer Foundation (AF-980907), the Swedish Brain Foundation (FO2021-0293), the Parkinson Foundation of Sweden (1412/22), the Cure Alzheimer’s Fund, the Rönström Family Foundation, the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, Skåne University Hospital Foundation (2020-O000028), the Regionalt Forskningsstöd (2022-1259) and the Swedish federal government under the ALF agreement (2022-Projekt0080). U.J.K. is supported by NIH grants R01NS131658, U01NS113851 and U01NS122419, R01NS133742 and U01NS126406. R.N.A. received funding from the Parkinson’s Foundation, the NIH, the Department of Defense, the MJFF, and the Silverstein Foundation for GBA/PD. D.S. is funded by Abbvie, the American Parkinson Disease Association, the MJFF, The National Parkinson Foundation, the Alabama Department of Commerce, the Alabama Innovation Fund, Genentech, the Department of Defense, and NIH grant P50NS108675. D.G. is funded by NIH grants P30AG062429 and U01NS100610. K.P. is a member of the executive steering committee of the Parkinson’s Progression Marker Initiative of the MJFF and has received funding from the MJFF and NIH (NS115114).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.S. is a founder, chief scientific officer, shareholder and member of the board of directors of Amprion, a biotechnology company that focuses on the commercial use of seed amplification assays for high-sensitivity detection of misfolded protein aggregates involved in various neurodegenerative diseases. The University of Texas Health Science Center has licensed patents to Amprion. B.M. is scientific advisor to Amprion and has received honoraria for consultancy and/or educational presentations from GE, Bial, Roche, Biogen and AbbVie. O.H. has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, C2N Diagnostics, Eli Lilly, Eisai, Fujirebio, GE Healthcare and Roche. In the past 2 years, he has received consultancy or speaker fees from AC Immune, Alzpath, BioArctic, Biogen, Bristol Meyer Squibb, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. U.J.K. is on the scientific advisory board of Amprion and consults for UCB, NurrOn and HanAll. R.N.A. has received consulting fees from Biogen, Biohaven, Capsida, Gain Therapeutics, Sanofi, Servier, Takeda and Vanqua Bio. D.S. is a consultant for or received honoraria from Abbvie, Alnylam Pharmaceutics, Appello, Biohaven Pharmaceuticals, BlueRock Therapeutics, Coave Therapeutics Curium Pharma, F. Hoffman-La Roche, Eli Lilly USA, Sanofi-Aventis and Theravance. K.M. declares support from his institution (Institute for Neurodegenerative Disorders) from the MJFF, and consultancies for Invicro, Xing Imaging, Ixico, the MJFF, Roche, Calico, Coave, Neuron23, Orbimed, Biohaven, Sanofi, Koneksa, Merck, Lilly, Inhibikase, Neuramedy, IRLabs and Prothena. D.G. has served as a consultant for GE Healthcare, Eisai and Biogen. K.P. is a scientific advisor to Amprion and has served as a consultant for Curasen, Novartis, Biohaven and Neuron23.
Peer review
Peer review information
Nature Medicine thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soto, C., Mollenhauer, B., Hansson, O. et al. Toward a biological definition of neuronal and glial synucleinopathies. Nat Med 31, 396–408 (2025). https://doi.org/10.1038/s41591-024-03469-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-024-03469-7
This article is cited by
-
PET imaging of alpha-synuclein: from radiotracer design through in vitro and in vivo translation
European Journal of Nuclear Medicine and Molecular Imaging (2026)
-
Alpha-synuclein amyloids catalyze the degradation of ATP and other nucleotides
Scientific Reports (2025)
-
A mechanistic model of pure and lipidic α-synuclein aggregation for advancing Parkinson’s therapies
Communications Chemistry (2025)
-
Revisiting Parkinson’s disease definition and classification: insights from two emerging biological frameworks
Journal of Neural Transmission (2025)
-
Cognitive and neuroimaging outcome of very prodromal dementia with Lewy bodies
GeroScience (2025)