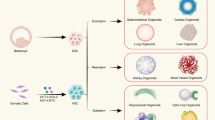
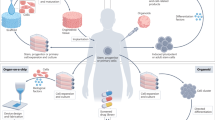
Abstract
Organoids are innovative three-dimensional and self-organizing cell cultures of various lineages that can be used to study diverse tissues and organs. Human organoids have dramatically increased our understanding of developmental and disease biology. They provide a patient-specific model to study known diseases, with advantages over animal models, and can also provide insights into emerging and future health threats related to climate change, zoonotic infections, environmental pollutants or even microgravity during space exploration. Furthermore, organoids show potential for regenerative cell therapies and organ transplantation. Still, several challenges for broad clinical application remain, including inefficiencies in initiation and expansion, increasing model complexity and difficulties with upscaling clinical-grade cultures and developing more organ-specific human tissue microenvironments. To achieve the full potential of organoid technology, interdisciplinary efforts are needed, integrating advances from biology, bioengineering, computational science, ethics and clinical research. In this Review, we showcase pivotal achievements in epithelial organoid research and technologies and provide an outlook for the future of organoids in advancing human health and medicine.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Marsee, A. et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 28, 816–832 (2021).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Spence, J. R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
Jiang, S. et al. Macrophage–organoid co-culture model for identifying treatment strategies against macrophage-related gemcitabine resistance. J. Exp. Clin. Cancer Res. 42, 199 (2023).
Zhou, G. et al. Modelling immune cytotoxicity for cholangiocarcinoma with tumour-derived organoids and effector T cells. Br. J. Cancer 127, 649–660 (2022).
Liu, J. et al. Cancer-associated fibroblasts provide a stromal niche for liver cancer organoids that confers trophic effects and therapy resistance. Cell. Mol. Gastroenterol. Hepatol. 11, 407–431 (2021).
Willemse, J., van der Laan, L. J. W., de Jonge, J. & Verstegen, M. M. A. Design by nature: emerging applications of native liver extracellular matrix for cholangiocyte organoid-based regenerative medicine. Bioengineering 9, 110 (2022).
van Tienderen, G. S. et al. Extracellular matrix drives tumor organoids toward desmoplastic matrix deposition and mesenchymal transition. Acta Biomater. 158, 115–131 (2023).
Ingber, D. E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 23, 467–491 (2022).
Matai, I., Kaur, G., Seyedsalehi, A., McClinton, A. & Laurencin, C. T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226, 119536 (2020).
Hendriks, D. et al. Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nat. Biotechnol. 41, 1567–1581 (2023).
Hendriks, D., Artegiani, B., Hu, H., Chuva de Sousa Lopes, S. & Clevers, H. Establishment of human fetal hepatocyte organoids and CRISPR–Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat. Protoc. 16, 182–217 (2021).
Sampaziotis, F. et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science 371, 839–846 (2021).
Ahn, S. J. Standards for organoids. Int. J. Stem Cells 17, 99–101 (2024).
Ahn, S. J. et al. Essential guidelines for manufacturing and application of organoids. Int. J. Stem Cells 17, 102–112 (2024).
Kelley, K. W. & Pașca, S. P. Human brain organogenesis: toward a cellular understanding of development and disease. Cell 185, 42–61 (2022).
Bombieri, C. et al. Advanced cellular models for rare disease study: exploring neural, muscle and skeletal organoids. Int. J. Mol. Sci. 25, 1014 (2024).
Okumuş, E. B., Böke, Ö. B., Turhan, S. & Doğan, A. From development to future prospects: the adipose tissue & adipose tissue organoids. Life Sci. 351, 122758 (2024).
Wang, X. H., Liu, N., Zhang, H., Yin, Z. S. & Zha, Z. G. From cells to organs: progress and potential in cartilaginous organoids research. J. Transl. Med. 21, 926 (2023).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988 (2018).
Schuth, S. et al. Patient-specific modeling of stroma-mediated chemoresistance of pancreatic cancer using a three-dimensional organoid–fibroblast co-culture system. J. Exp. Clin. Cancer Res. 41, 312 (2022).
Strating, E. et al. Co-cultures of colon cancer cells and cancer-associated fibroblasts recapitulate the aggressive features of mesenchymal-like colon cancer. Front. Immunol. 14, 1053920 (2023).
Homan, K. A. et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262 (2019).
Willemse, J. et al. Scaffolds obtained from decellularized human extrahepatic bile ducts support organoids to establish functional biliary tissue in a dish. Biotechnol. Bioeng. 118, 836–851 (2021).
Rüland, L. et al. Organoid models of fibrolamellar carcinoma mutations reveal hepatocyte transdifferentiation through cooperative BAP1 and PRKAR2A loss. Nat. Commun. 14, 2377 (2023).
Lawlor, K. T. et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat. Mater. 20, 260–271 (2021).
Bernal, P. N. et al. Volumetric bioprinting of organoids and optically tuned hydrogels to build liver-like metabolic biofactories. Adv. Mater. 34, e2110054 (2022).
Wang, X., Luo, Y., Ma, Y., Wang, P. & Yao, R. Converging bioprinting and organoids to better recapitulate the tumor microenvironment. Trends Biotechnol. 42, 648–663 (2024).
Chen, H. et al. Acoustic bioprinting of patient-derived organoids for predicting cancer therapy responses. Adv. Healthc. Mater. 11, e2102784 (2022).
Brassard, J. A., Nikolaev, M., Hübscher, T., Hofer, M. & Lutolf, M. P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat. Mater. 20, 22–29 (2021).
Urciuolo, A. et al. Hydrogel-in-hydrogel live bioprinting for guidance and control of organoids and organotypic cultures. Nat. Commun. 14, 3128 (2023).
Yarali, E. et al. 4D printing for biomedical applications. Adv. Mater. 36, e2402301 (2024).
Chen, A. et al. Multimaterial 3D and 4D bioprinting of heterogenous constructs for tissue engineering. Adv. Mater. 36, e2307686 (2023).
Chadwick, M. et al. Rapid processing and drug evaluation in glioblastoma patient-derived organoid models with 4D bioprinted arrays. iScience 23, 101365 (2020).
Schuster, B. et al. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun. 11, 5271 (2020).
Shi, H. et al. Organoid intelligence: integration of organoid technology and artificial intelligence in the new era of in vitro models. Med. Nov. Technol. Devices 21, 100276 (2024).
Bai, L. et al. AI-enabled organoids: construction, analysis, and application. Bioact. Mater. 31, 525–548 (2024).
Du, X. et al. Organoids revealed: morphological analysis of the profound next generation in-vitro model with artificial intelligence. Biodes. Manuf. 6, 319–339 (2023).
Fujii, M. & Sato, T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat. Mater. 20, 156–169 (2021).
Viergever, B. J. et al. Urine-derived bladder cancer organoids (urinoids) as a tool for cancer longitudinal response monitoring and therapy adaptation. Br. J. Cancer 130, 369–379 (2024).
Sun, G. et al. Formation and optimization of three-dimensional organoids generated from urine-derived stem cells for renal function in vitro. Stem Cell Res. Ther. 11, 309 (2020).
Soroka, C. J. et al. Bile-derived organoids from patients with primary sclerosing cholangitis recapitulate their inflammatory immune profile. Hepatology 70, 871–882 (2019).
Roos, F. J. M. et al. Human bile contains cholangiocyte organoid-initiating cells which expand as functional cholangiocytes in non-canonical Wnt stimulating conditions. Front. Cell Dev. Biol. 8, 630492 (2021).
Gerli, M. F. M. et al. Single-cell guided prenatal derivation of primary fetal epithelial organoids from human amniotic and tracheal fluids. Nat. Med. 30, 875–887 (2024).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Fujii, M. et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838 (2016).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Boj, S. F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338 (2015).
Nanki, K. et al. Somatic inflammatory gene mutations in human ulcerative colitis epithelium. Nature 577, 254–259 (2020).
Nanki, K. et al. Divergent routes toward Wnt and R-spondin niche independency during human gastric carcinogenesis. Cell 174, 856–869 (2018).
Broutier, L. et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 12, 1424–1435 (2017).
Saito, Y. et al. Establishment of patient-derived organoids and drug screening for biliary tract carcinoma. Cell Rep. 27, 1265–1276 (2019).
Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386 (2018).
Kawasaki, K. et al. An organoid biobank of neuroendocrine neoplasms enables genotype–phenotype mapping. Cell 183, 1420–1435 (2020).
Pauli, C. et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 7, 462–477 (2017).
Tiriac, H. et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 8, 1112–1129 (2018).
van Tienderen, G. S. et al. Hepatobiliary tumor organoids for personalized medicine: a multicenter view on establishment, limitations, and future directions. Cancer Cell 40, 226–230 (2022).
Seino, T. et al. Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression. Cell Stem Cell 22, 454–467 (2018).
Abaza, A. et al. Programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) immunotherapy: a promising breakthrough in cancer therapeutics. Cureus 15, e44582 (2023).
Shiravand, Y. et al. Immune checkpoint inhibitors in cancer therapy. Curr. Oncol. 29, 3044–3060 (2022).
Sharma, P. et al. Immune checkpoint therapy—current perspectives and future directions. Cell 186, 1652–1669 (2023).
Sharma, P. & Allison, J. P. Dissecting the mechanisms of immune checkpoint therapy. Nat. Rev. Immunol. 20, 75–76 (2020).
Sleeboom, J. J. F. et al. The extracellular matrix as hallmark of cancer and metastasis: from biomechanics to therapeutic targets. Sci. Transl. Med. 16, eadg3840 (2024).
Dijkstra, K. K. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598 (2018).
Magré, L. et al. Emerging organoid–immune co-culture models for cancer research: from oncoimmunology to personalized immunotherapies. J. Immunother. Cancer 11, e006290 (2023).
van Tienderen, G., Groot Koerkamp, B., IJzermans, J., van der Laan, L. & Verstegen, M. Recreating tumour complexity in a dish: organoid models to study liver cancer cells and their extracellular environment. Cancers 11, 1706 (2019).
van Tienderen, G. S. et al. Modelling metastatic colonization of cholangiocarcinoma organoids in decellularized lung and lymph nodes. Front. Oncol. 12, 1101901 (2022).
Polak, R., Zhang, E. T. & Kuo, C. J. Cancer organoids 2.0: modelling the complexity of the tumour immune microenvironment. Nat. Rev. Cancer 24, 523–539 (2024).
LeSavage, B. L., Suhar, R. A., Broguiere, N., Lutolf, M. P. & Heilshorn, S. C. Next-generation cancer organoids. Nat. Mater. 21, 143–159 (2022).
Rosendahl Huber, A. et al. Improved detection of colibactin-induced mutations by genotoxic E. coli in organoids and colorectal cancer. Cancer Cell 42, 487–496 (2024).
Kwong, J. et al. Inflammatory cytokine tumor necrosis factor α confers precancerous phenotype in an organoid model of normal human ovarian surface epithelial cells. Neoplasia 11, 529–541 (2009).
Kopper, O. et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat. Med. 25, 838–849 (2019).
Kessler, M. et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 6, 8989 (2015).
Hoffmann, K. et al. Stable expansion of high-grade serous ovarian cancer organoids requires a low-Wnt environment. EMBO J. 39, e104013 (2020).
Turco, M. Y. et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 19, 568–577 (2017).
Boretto, M. et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 144, 1775–1786 (2017).
Fitzgerald, H. C., Dhakal, P., Behura, S. K., Schust, D. J. & Spencer, T. E. Self-renewing endometrial epithelial organoids of the human uterus. Proc. Natl Acad. Sci. USA 116, 23132–23142 (2019).
Chumduri, C. et al. Opposing Wnt signals regulate cervical squamocolumnar homeostasis and emergence of metaplasia. Nat. Cell Biol. 23, 184–197 (2021).
Lõhmussaar, K. et al. Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell 28, 1380–1396 (2021).
Maru, Y. et al. Establishment and molecular phenotyping of organoids from the squamocolumnar junction region of the uterine cervix. Cancers 12, 694 (2020).
Turco, M. Y. et al. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 564, 263–267 (2018).
Haider, S. et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports 11, 537–551 (2018).
Alzamil, L., Nikolakopoulou, K. & Turco, M. Y. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 28, 35–51 (2021).
Karthaus, W. R. et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159, 163–175 (2014).
Baert, Y. et al. Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Reports 8, 30–38 (2017).
Beshiri, M., Agarwal, S., Yin, J. J. & Kelly, K. Prostate organoids: emerging experimental tools for translational research. J. Clin. Invest. 133, e169616 (2023).
Calà, G., Sina, B., De Coppi, P., Giobbe, G. G. & Gerli, M. F. M. Primary human organoids models: current progress and key milestones. Front. Bioeng. Biotechnol. 11, 1058970 (2023).
Bianchi, D. W. From prenatal genomic diagnosis to fetal personalized medicine: progress and challenges. Nat. Med. 18, 1041–1051 (2012).
de Coppi, P. et al. Regenerative medicine: prenatal approaches. Lancet Child Adolesc. Health 6, 643–653 (2022).
Deprest, J. A. et al. Randomized trial of fetal surgery for moderate left diaphragmatic hernia. N. Engl. J. Med. 385, 119–129 (2021).
Deprest, J. A. et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N. Engl. J. Med. 385, 107–118 (2021).
Zani, A. et al. Congenital diaphragmatic hernia. Nat. Rev. Dis. Primers 8, 37 (2022).
Cheng, P. P. et al. PM2.5 exposure-induced senescence-associated secretory phenotype in airway smooth muscle cells contributes to airway remodeling. Environ. Pollut. 347, 123674 (2024).
Winkler, A. S. et al. Human airway organoids and microplastic fibers: a new exposure model for emerging contaminants. Environ. Int. 163, 107200 (2022).
Zhou, Y. et al. Low-dose of polystyrene microplastics induce cardiotoxicity in mice and human-originated cardiac organoids. Environ. Int. 179, 108171 (2023).
Cheng, W. et al. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 806, 150328 (2022).
Astashkina, A. I. et al. Nanoparticle toxicity assessment using an in vitro 3-D kidney organoid culture model. Biomaterials 35, 6323–6331 (2014).
Williams, K. E. et al. Quantitative proteomic analyses of mammary organoids reveals distinct signatures after exposure to environmental chemicals. Proc. Natl Acad. Sci. USA 113, E1343–E1351 (2016).
Shaoyong, W. et al. Benzo [a] pyrene-loaded aged polystyrene microplastics promote colonic barrier injury via oxidative stress-mediated Notch signalling. J. Hazard. Mater. 457, 131820 (2023).
Zhang, C. et al. Inhibition of GABAA receptors in intestinal stem cells prevents chemoradiotherapy-induced intestinal toxicity. J. Exp. Med. 219, e20220541 (2022).
Cheng, W. et al. The iron matters: aged microplastics disrupted the iron homeostasis in the liver organoids. Sci. Total Environ. 906, 167529 (2024).
Xuan, L. et al. Predictive metabolomic signatures for safety assessment of three plastic nanoparticles using intestinal organoids. Sci. Total Environ. 913, 169606 (2024).
Loiseau, C. & Sorci, G. Can microplastics facilitate the emergence of infectious diseases? Sci. Total Environ. 823, 153694 (2022).
Nii-Trebi, N. I. et al. Dynamics of viral disease outbreaks: a hundred years (1918/19–2019/20) in retrospect — loses, lessons and emerging issues. Rev. Med. Virol. 33, e2475 (2023).
Ettayebi, K. et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 353, 1387–1393 (2016).
Garcez, P. P. et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816–818 (2016).
Karvas, R. M. et al. Stem-cell-derived trophoblast organoids model human placental development and susceptibility to emerging pathogens. Cell Stem Cell 29, 810–825 (2022).
Wu, H. et al. Zika virus targets human trophoblast stem cells and prevents syncytialization in placental trophoblast organoids. Nat. Commun. 14, 5541 (2023).
Lamers, M. M. et al. An organoid-derived bronchioalveolar model for SARS-CoV-2 infection of human alveolar type II-like cells. EMBO J. 40, e105912 (2021).
Jansen, J. et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell 29, 217–231 (2022).
Zhao, B. et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 11, 771–775 (2020).
Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54 (2020).
Li, P. et al. Mpox virus infection and drug treatment modelled in human skin organoids. Nat. Microbiol. 8, 2067–2079 (2023).
Li, P. et al. Mpox virus infects and injures human kidney organoids, but responding to antiviral treatment. Cell Discov. 9, 34 (2023).
Merad, M. & Martin, J. C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 20, 355–362 (2020).
Xu, M. et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 22, 1101–1107 (2016).
Han, Y. et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 589, 270–275 (2021).
Li, P. et al. Recapitulating hepatitis E virus–host interactions and facilitating antiviral drug discovery in human liver-derived organoids. Sci. Adv. 8, eabj5908 (2022).
Wagar, L. E. et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 27, 125–135 (2021).
Zhou, J. et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 26, 1077–1083 (2020).
Partridge, L., Deelen, J. & Slagboom, P. E. Facing up to the global challenges of ageing. Nature 561, 45–56 (2018).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Blokzijl, F. et al. Tissue-specific mutation accumulation in human adult stem cells during life. Nature 538, 260–264 (2016).
Vougioukalaki, M. et al. Different responses to DNA damage determine ageing differences between organs. Aging Cell 21, e13562 (2022).
Nguyen, L. et al. Precancerous liver diseases do not cause increased mutagenesis in liver stem cells. Commun. Biol. 4, 1301 (2021).
Pentinmikko, N. et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 571, 398–402 (2019).
Kobayashi, Y. et al. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat. Cell Biol. 22, 934–946 (2020).
Nalapareddy, K. et al. Canonical Wnt signaling ameliorates aging of intestinal stem cells. Cell Rep. 18, 2608–2621 (2017).
Ferreira-Gonzalez, S. et al. Senolytic treatment preserves biliary regenerative capacity lost through cellular senescence during cold storage. Sci. Transl. Med. 14, eabj4375 (2022).
Krittanawong, C. et al. Human health during space travel: state-of-the-art review. Cells 12, 40 (2022).
Garrett-Bakelman, F. E. et al. The NASA Twins Study: a multidimensional analysis of a year-long human spaceflight. Science 364, eaau8650 (2019).
Kirkpatrick, A. W. et al. Do we have the guts to go? The abdominal compartment, intra-abdominal hypertension, the human microbiome and exploration class space missions. Can. J. Surg. 63, E581–E593 (2020).
Crucian, B. et al. Incidence of clinical symptoms during long-duration orbital spaceflight. Int. J. Gen. Med. 9, 383–391 (2016).
Turroni, S. et al. Gut microbiome and space travelers’ health: state of the art and possible pro/prebiotic strategies for long-term space missions. Front. Physiol. 11, 553929 (2020).
Mercadante, V. et al. Salivary gland hypofunction and/or xerostomia induced by nonsurgical cancer therapies: ISOO/MASCC/ASCO guideline. J. Clin. Oncol. 39, 2825–2843 (2021).
Cinat, D., Coppes, R. P. & Barazzuol, L. DNA damage-induced inflammatory microenvironment and adult stem cell response. Front. Cell Dev. Biol. 9, 729136 (2021).
van Luijk, P. et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Sci. Transl. Med. 7, 305ra147 (2015).
Maimets, M. et al. Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Reports 6, 150–162 (2016).
Pringle, S. et al. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells 34, 640–652 (2016).
Zanten, J. V. et al. Optimization of the production process of clinical-grade human salivary gland organoid-derived cell therapy for the treatment of radiation-induced xerostomia in head and neck cancer. Pharmaceutics 16, 435 (2024).
Gutiérrez, E. & Andrés, A. Selection of donor and organ viability criteria: expanding donation criteria. J. Ren. Care 33, 83–88 (2007).
Lascaris, B., de Meijer, V. E. & Porte, R. J. Normothermic liver machine perfusion as a dynamic platform for regenerative purposes: what does the future have in store for us? J. Hepatol. 77, 825–836 (2022).
Schurink, I. J., de Jonge, J. & van der Laan, L. J. W. Organoid technology starts to deliver: repairing damaged liver grafts during normothermic machine perfusion. Transplantation 105, 1886–1887 (2021).
Levink, I. J. M. et al. Optimization of pancreatic juice collection: a first step toward biomarker discovery and early detection of pancreatic cancer. Am. J. Gastroenterol. 115, 2103–2108 (2020).
Guo, H. et al. 3-D human renal tubular organoids generated from urine-derived stem cells for nephrotoxicity screening. ACS Biomater. Sci. Eng. 6, 6701–6709 (2020).
Schneeberger, K. et al. Large-scale production of LGR5-positive bipotential human liver stem cells. Hepatology 72, 257–270 (2020).
Madl, C. M., Heilshorn, S. C. & Blau, H. M. Bioengineering strategies to accelerate stem cell therapeutics. Nature 557, 335–342 (2018).
Below, C. R. et al. A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nat. Mater. 21, 110–119 (2022).
Gnecco, J. S. et al. Organoid co-culture model of the human endometrium in a fully synthetic extracellular matrix enables the study of epithelial–stromal crosstalk. Med 4, 554–579 (2023).
Gjorevski, N. et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016).
Willemse, J. et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials 284, 121473 (2022).
Martinez-Garcia, F. D. et al. Architecture and composition dictate viscoelastic properties of organ-derived extracellular matrix hydrogels. Polymers 13, 3113 (2021).
Liguori, G. R. et al. Molecular and biomechanical clues from cardiac tissue decellularized extracellular matrix drive stromal cell plasticity. Front. Bioeng. Biotechnol. 8, 520 (2020).
Elomaa, L., Keshi, E., Sauer, I. M. & Weinhart, M. Development of GelMA/PCL and dECM/PCL resins for 3D printing of acellular in vitro tissue scaffolds by stereolithography. Mater. Sci. Eng. C Mater. Biol. Appl. 112, 110958 (2020).
de Jongh, D., Massey, E. K., consortium, V. & Bunnik, E. M. Organoids: a systematic review of ethical issues. Stem Cell Res. Ther. 13, 337 (2022).
Barnhart, A. J. & Dierickx, K. The many moral matters of organoid models: a systematic review of reasons. Med. Health Care Philos. 25, 545–560 (2022).
Bredenoord, A. L., Clevers, H. & Knoblich, J. A. Human tissues in a dish: the research and ethical implications of organoid technology. Science 355, eaaf9414 (2017).
Hyun, I., Scharf-Deering, J. C. & Lunshof, J. E. Ethical issues related to brain organoid research. Brain Res. 1732, 146653 (2020).
de Graeff, N., De Proost, L. & Munsie, M. ‘Ceci n’est pas un embryon?’ The ethics of human embryo model research. Nat. Methods 20, 1863–1867 (2023).
Jongsma, K. R. & Bredenoord, A. L. Ethics parallel research: an approach for (early) ethical guidance of biomedical innovation. BMC Med. Ethics 21, 81 (2020).
Kramer, K. When is something an alternative? A general account applied to animal-free alternatives to animal research. Camb. Q. Healthc. Ethics 33, 89–101 (2024).
Boers, S. N. & Bredenoord, A. L. Consent for governance in the ethical use of organoids. Nat. Cell Biol. 20, 642–645 (2018).
Lensink, M. A. et al. Responsible use of organoids in precision medicine: the need for active participant involvement. Development 147, dev177972 (2020).
Boers, S. N., van Delden, J. J., Clevers, H. & Bredenoord, A. L. Organoid biobanking: identifying the ethics: organoids revive old and raise new ethical challenges for basic research and therapeutic use. EMBO Rep. 17, 938–941 (2016).
Seah, C. & Brennand, K. J. Pandemic city: village-in-a-dish unlocks dynamic genetic effects in the brain. Cell Stem Cell 30, 239–241 (2023).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Karuna Ganesh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verstegen, M.M.A., Coppes, R.P., Beghin, A. et al. Clinical applications of human organoids. Nat Med 31, 409–421 (2025). https://doi.org/10.1038/s41591-024-03489-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41591-024-03489-3
This article is cited by
-
3D tumor cultures for drug resistance and screening development in clinical applications
Molecular Cancer (2025)
-
Glioblastoma metabolomics: uncovering biomarkers for diagnosis, prognosis and targeted therapy
Journal of Experimental & Clinical Cancer Research (2025)
-
Establishment and evaluation of a patient-derived organoids-based xenograft model of primary aldosteronism using [18F]AlF-NOTA-pentixather PET/CT: bridging preclinical and clinical imaging
Journal of Translational Medicine (2025)
-
Three-dimensional midbrain organoids: a next-generation tool for Parkinson’s disease modelling and drug discovery
Stem Cell Research & Therapy (2025)
-
Bioelectronic Interfaces and Sensors for Neural Organoids
Microsystems & Nanoengineering (2025)