Abstract
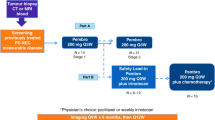
Historically, the treatment of patients with advanced stage or recurrent endometrial cancer included paclitaxel plus carboplatin. Immunotherapy in combination with chemotherapy resulted in improved clinical outcomes in several solid tumors. In the phase 3 NRG GY018 study, pembrolizumab plus chemotherapy significantly improved investigator-assessed progression-free survival (PFS; primary endpoint) versus placebo plus chemotherapy in patients with advanced/metastatic/recurrent endometrial cancer regardless of mismatch repair status. Here we report on key secondary endpoints and exploratory analyses. Patients were women ≥18 years old with newly diagnosed stage III or IVA endometrial cancer with measurable disease, or stage IVB or recurrent endometrial cancer with or without measurable disease. Patients (n = 810) were randomized (1:1) to pembrolizumab or placebo plus paclitaxel–carboplatin followed by maintenance pembrolizumab or placebo for up to 24 months. Overall survival was a secondary endpoint and PFS per RECIST v.1.1 by blinded independent central review was an exploratory endpoint. Overall survival data were immature; hazard ratios favored pembrolizumab (mismatch repair-proficient: 0.79 (0.53–1.17); 1-sided nominal P = 0.1157; mismatch repair-deficient: 0.55 (0.25–1.19); 1-sided nominal P = 0.0617). Hazard ratios (95% confidence intervals) for PFS per blinded independent central review favored pembrolizumab (mismatch repair-proficient: 0.64 (0.49–0.85); P = 0.0008; mismatch repair-deficient: 0.45 (0.27–0.73); P = 0.0005). These findings further support the use of pembrolizumab plus chemotherapy as first-line treatment for patients with advanced stage or recurrent endometrial cancer regardless of mismatch repair status. ClinicalTrials.gov identifier: NCT03914612.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at https://externaldatasharing-msd.com/) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
References
Mamat Yusof, M. N. et al. Efficacy and safety of PD-1/PD-L1 inhibitor as single-agent immunotherapy in endometrial cancer: a systematic review and meta-analysis. Cancers 15, 4032 (2023).
Bartoletti, M. et al. Incorporation of anti-PD1 or anti PD-L1 agents to platinum-based chemotherapy for the primary treatment of advanced or recurrent endometrial cancer. A meta-analysis. Cancer Treat. Rev. 125, 102701 (2024).
O’Malley, D. M. et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J. Clin. Oncol. 40, 752–761 (2022).
Makker, V. et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N. Engl. J. Med. 386, 437–448 (2022).
Makker, V. et al. Lenvatinib plus pembrolizumab in previously treated advanced endometrial cancer: updated efficacy and safety from the randomized phase III study 309/KEYNOTE-775. J. Clin. Oncol. 41, 2904–2910 (2023).
Keytruda (Pembrolizumab) (US Food and Drug Administration, 2024); www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
Summary of Product Characteristics. Keytruda (Pembrolizumab) (European Medicines Agency, 2024); www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf
Eskander, R. N. et al. Pembrolizumab plus chemotherapy in advanced endometrial cancer. N. Engl. J. Med. 388, 2159–2170 (2023).
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Uterine Neoplasms v.2. (National Comprehensive Cancer Network (NCCN), 2024).
Tempfer, C. et al. Statement of the Uterus Commission of the Gynecological Oncology Working Group (AGO) on the use of primary chemoimmunotherapy to treat patients with locally advanced or recurrent endometrial cancer. Geburtshilfe Frauenheilkd. 83, 1095–1101 (2023).
Powell, M. A. et al. Dostarlimab for primary advanced or recurrent (A/R) endometrial cancer (EC): outcomes by blinded independent central review (BICR) of the RUBY trial (ENGOT-EN6-NSGO/GOG-3031/RUBY). J. Clin. Oncol. 41, 5503 (2023).
Westin, S. N. et al. Durvalumab plus carboplatin/paclitaxel followed by maintenance durvalumab with or without olaparib as first-line treatment for advanced endometrial cancer: the phase III DUO-E trial. J. Clin. Oncol. 42, 283–299 (2024).
Acknowledgements
This work was supported by grants U10CA180868 and U10CA180822 from the National Cancer Institute (NCI). Funding and support were received from Merck & Co., Inc., Rahway, NJ, USA through a Cooperative Research and Developmental Agreement with NCI. Merck & Co., Inc. also provided supplemental funding to NRG Oncology for this study. Support was provided in part by the National Institutes of Health (NIH)/NCI Cancer Center Support Grant P30CA008748 (to C.A. and R.E.O.C.) and NIH-UG1 CA233330 (to C.A.L.). Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. (MSD). The protocol was developed by NRG Oncology. MSD had a role in the current data analysis, the decision to publish and paper review. We thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. Medical writing assistance was provided by M. S. McNamara of ICON plc (Blue Bell, PA, USA). This assistance was funded by MSD. The following NRG Oncology Group member institutions participated in the primary treatment studies: CWRU Case Comprehensive Cancer Center, University of Rochester, Pacific Cancer Research Consortium, University of Oklahoma Health Sciences Center, Thomas Jefferson University Hospital, Georgia NCI Community Oncology Research Program, Rutgers Cancer Institute of New Jersey, Women and Infants Hospital, Kaiser Permanente NCI Community Oncology Research Program, University of Alabama at Birmingham/Deep South Research Consortium, Odette Cancer Centre – Sunnybrook Health Sciences Centre, Northwestern University, Roswell Park Comprehensive Cancer Center, Indiana University/Melvin and Bren Simon Cancer Center, Ohio State University Comprehensive Cancer Center, Memorial Sloan-Kettering Cancer Center, Michigan Cancer Research Consortium NCORP, University of Iowa/Holden Comprehensive Cancer Center, Washington University – Siteman Cancer Center, Northwell Health NCORP, UC San Diego Moores Cancer Center, CommonSpirit Health Research Institute, NorthShore University HealthSystem at Evanston Hospital, University of Michigan Comprehensive Cancer Center, UPMC Hillman Cancer Center, ProMedica Flower Hospital, Sanford NCI Community Oncology Research Program of the North Central Plains, West Virginia University Charleston Division, Nebraska Methodist Hospital, Mayo Clinic, University of Nebraska Medical Center, University of Cincinnati Cancer Center – UC Medical Center, Houston Methodist Hospital, Cancer Research Consortium of West Michigan NCORP, University of Kansas Cancer Center – MCA Rural MU NCORP, Greater Baltimore Medical Center, Carolinas Medical Center/Levine Cancer Institute, New Mexico Minority Underserved NCORP, Avera Cancer Institute, University of Virginia Cancer Center, Legacy Good Samaritan Hospital and Medical Center, Saitama Medical University International Medical Center, Columbus NCI Community Oncology Research Program, JHU Sidney Kimmel Comprehensive Cancer Center, Puerto Rico Minority Underserved NCORP, West Virginia University Healthcare, Mount Sinai Hospital, UC Irvine Health/Chao Family Comprehensive Cancer Center, Lahey Hospital and Medical Center, Montana Cancer Consortium NCORP, Allegheny General Hospital, VCU Massey Cancer Center Minority Underserved NCORP, CHUM – Centre hospitalier de l’Université de Montréal, University Health Network – Princess Margaret Hospital, UCLA/Jonsson Comprehensive Cancer Center, Gulf South Minority Underserved NCORP, Heartland Cancer Research NCORP, Yale University – Yale Cancer Center LAPS, Montefiore Minority Underserved NCORP, Wake Forest University Health Sciences, New York-Presbyterian/Brooklyn Methodist Hospital, Sutter Cancer Research Consortium, UNC Lineberger Comprehensive Cancer Center LAPS, Cancer Research for the Ozarks NCORP, WellSpan Health – York Hospital, Southeast Clinical Oncology Research Consortium NCORP, Baylor College of Medicine/Dan L. Duncan Comprehensive Cancer Center, Saskatoon Cancer Centre, Juravinski Cancer Centre at Hamilton Health Sciences, Aurora NCI Community Oncology Research Program, University of Illinois, University of Chicago Comprehensive Cancer Center, Medical University of South Carolina Minority Underserved NCORP, University of Pennsylvania/Abramson Cancer Center, Wisconsin NCI Community Oncology Research Program, Cedars-Sinai Medical Center, Danbury Hospital, Midwestern Regional Medical Center, Seoul National University Hospital, Carle Cancer Center NCI Community Oncology Research Program, Geisinger Cancer Institute NCI Community Oncology Research Program, Winship Cancer Institute of Emory University, Dana-Farber/Partners Cancer Care, Dartmouth College – Norris Cotton Cancer Center, University of Utah – Huntsman Cancer Institute, University of Wisconsin Carbone Cancer Center, Stony Brook University Medical Center, Vanderbilt University – Ingram Cancer Center, Baystate Medical Center, University of Mississippi Medical Center, Allan Blair Cancer Centre, City of Hope Comprehensive Cancer Center, UCSF Medical Center – Mount Zion, Delaware/Christiana Care NCI Community Oncology Research Program, Hartford Hospital, Hawaii Minority Underserved NCORP, Loyola University Medical Center, Rush University Medical Center, University of Colorado Cancer Center, University of Texas Southwestern Medical Center, UMass Memorial Medical Center – Memorial Division, Maine Health Cancer Care Network, Nevada Cancer Research Foundation NCORP, Cooper Hospital University Medical Center and Mercy Cancer Center – Sacramento and Miami Valley Hospital.
Author information
Authors and Affiliations
Contributions
The study concept and design was provided by R.N.E., M.W.S., C.A., A.N.F. and M.A.P. The provision of materials or patients is accredited to R.N.E., C.A., L.B., R.G.M., J.M.H., F.B.M., R.S.M., M.S.S., G.H.C., E.G., E.L., J.K., C.A.L., L.T.G., E.M.H., S.B.L, L.M.L., F.B., R.E.O.C., T.A.B., E.K.H., P.H.T., V.S.J., S.W., A.N.F. and M.A.P. Data acquisition was provided by R.N.E., M.W.S., C.A., R.G.M., J.M.H., F.B.M., R.S.M., M.S.S., E.G., J.K., C.A.L., E.M.H., S.B.L., R.E.O.C., A.N.F. and M.A.P. Data analysis, interpretation and verification are accredited to R.N.E., C.A., R.S.M., M.S.S., G.H.C., E.G., R.E.O.C. and A.N.F. We thank R.N.E., C.A., R.S.M., E.G., L.M.L., P.H.T. and A.N.F. for writing the paper. All authors contributed to the critical review of the paper and the final approval.
Corresponding author
Ethics declarations
Competing interests
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc. (MSD). The protocol was developed by NRG Oncology. MSD had a role in the current data analysis, the decision to publish and manuscript review. R.N.E. received institution research support from AstraZeneca, Clovis Oncology, Eisai, MSD and Novocure; consulting and advisory fees from AstraZeneca, Cardiff Oncology, Clovis Oncology, Eisai, Elevar Therapeutics, GSK/Tesaro, ImmunoGen, Inc., Seagen, Mersana, Myriad, Daichi Sankyo, PMV, Gilead, Onconova, Elevar Therapeutics and Regeneron along with personal payments for lectures and presentations from AstraZeneca, GSK, Immunogen, Merck, Medscape, Curio Biosciences, Great Debates and Updates. Additionally, R.N.E. serves in a leadership role as GOG Associate Clinical Trial Advisor as well as Scientific and Medical Advisor to the Clearity Foundation. M.W.S. reports receiving support from his employer, the Roswell Park Comprehensive Cancer Center with support from the National Cancer Institute as the government agency that funds the research. M.W.S. also acknowledges receiving support to attend meetings and travel R.G.M. received consulting fees from Fujirebio Diagnostics, Inc. F.B.M. received institutional support for clinical trials from GSK, Clovis, Immunogen, AstraZeneca, Seagen, Duality Bio, Acrivon, Allarity and Adaptimune, as well as consulting fees from the advisory boards of AstraZeneca, GSK, Daiichi Sankyo, BioNTech, Aadi. Consulting fees were also received from GSK and Seagen and an additional speaker fee from MSD. F.B.M. paritcipated on advisory boards for AstraZeneca, GSK, Daiichi Sankyo, BioNTech and Aadi and also reports serving on the Board of Directors for the Society of Gynecologic Oncology (unpaid) as well as on the Board of Directors for the Rivkin Foundation (uncompensated). R.S.M. serves on the NRG Board of Directors for which his Institution receives compensation. M.S.S. receives consulting fees and honoraria from GSK, AstraZeneca, Merck and Immunogen and also serves as a board member for Unite for HER, a non-profit organization. E.G. served on an advisory board for Merck. E.L. received a grant from the Rhode Island Foundation. J.K. reports that US$300 was paid to their institution, Kaiser Permanente, in March 2023 for a lecture to the Association of Northern California Oncologists (ANCO) as well as receiving hotel expenses for this meeting. J.K. participated on an advisory board for a mirvetuximab study and approximately US$600 was paid to the institution in May 2022. C.A.L. received research funding for their institution and personal consulting fees, honorarium and international travel expenses from Merck. L.T.G. was paid consulting fees from Merck and GSK and also paid honoraria by AstraZeneca as a speaker. E.M.H. received consulting fees from AbbVie/Immunogen. L.M.L. received consulting fees for her participation on GlaxoSmithKline’s advisory board. Research grants were paid to the institution of F.B. from Merck, Eisai, ImmunoGen, Clovis, Beigene, Natera, Tempus and AstraZeneca while personal fees were also obtained from UptoDate and advisory boards (personal fees) were received from Agenus, Merck, Clovis, Immunogen, Eisai, AstraZeneca, GlaxoSmithKline, Myriad, BioNTech, Daiichi Sankyo, EMD Serono. Personal fees were also received by F.B. for CME lectures from Clinical Educational Concepts, Clinical Care Options, Medscape/WebMD, Med Learning, I3Health, CMR Institute, Global Learning Initiative/Prova, OncLive, Targeted Oncology, Research to Practice, GOG Foundation. GlaxoSmithKline and BioNTech provided support for F.B. to attend meetings and travel. F.B. holds uncompensated leadership roles on the Society of Gynecologic Oncology Board Member as a Co-Chair; NRG Oncology Developmental Therapeutic as a committee member and IGCS Education 360 as a Co-Chair. R.E.O.C. had support for this paper from NCI/NIH (P30CA008748). Grants were paid to R.E.O.C.’s institution from Bayer/Celgene/Juno, Arsenalbio, Tesaro/GSK, Merck, Ludwig Cancer Institute, AbbVie/StemCentrx, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, Inc., Marker Therapeutics, Syndax Pharmaceuticals, Genmab/Seagen Therapeutics, Genentech, Alkermes/Mural Oncology, Kite Pharma, Acrivon, OnCusp Therapeutics, and Gynecologic Oncology Foundation and the Lyell Immunopharma. R.E.O.C. also received payment and honoraria (one-off lectures) from GSK, Curio/Onclive/PER/MJH/Aptitude Health; SITC; Gynecologic Oncology Canada along with support for attending meetings from Hitech Health; Gathering Around Cancer, Ireland; GOG Foundation and SGO. In addition, R.E.O.C. served in an unpaid capacity for the Steering Committee for AstraZeneca (DUO-0), GSK (Moonstone, Prima) and Acrivon, as well as an unpaid advisor for Carina Biotech and Link Therapeutics. Furthermore, R.E.O.C. was an advisor to OnCusp Therapeutics and served on advisory boards for Tesaro/GSK, Regeneron Advisory, Seattle Genetics/Seagen, Immunogen Board Bayer, R Pharm, Fresenius Kabi, Miltenyi, 2seventy bio, Bayer and Loxo. R.E.O.C. is Vice-Chair for CPC and SGO and Chair of the DT Committee for NRG Oncology. The institution of P.H.T. received grants from Merck and GlaxoSmithKline. P.H.T. has stock in Immunon and was personally compensated for serving on advisory boards for Iovance, AstraZeneca, Verastem, GlaxoSmithKline, Seagen, Immunon, Immunogen, Mersana, Novocure, Zentalis, Caris, Merck and Caris. S.W. received personal payments and honoraria from Merck, GSK, AstraZeneca and Eisai. A.N.F. received honararia for speaking at the GOG Foundation, Endometrial Cancer Symposium and has also participated on the GSK advisory board while also serving as President of the Society of Gynecologic Oncology (unpaid position). M.A.P. received personal consulting fees from GSK, Tesaro, Merck, AstraZeneca, Immunogen, Seagen, Clovis Oncology and Kyropharma. M.A.P. also served in leadership roles for the GOG Foundation, SGO and FWC (uncompensated) alongside his role with NRG Oncology (compensated). C.A. received clinical trial funding to their institution (MSK) from AbbVie – MSK PI, GOG3005, Artios Pharma – International Coordinating Investigator & MSK PI, Artist; AstraZeneca – MSK PI, SOLO1/GOG 3004; National Coordinating Investigator & MSK PI, D081RC00001; ENGOT – ov46; AGO-OVAR 23; GOG-3025; Clovis – MSK PI, ARIEL 2 & 3; Genentech/Roche – MSK PI, GOG3015 (imAGYN050). C.A. participated in a virtual advisory board for Blueprint Medicine on 30 June 2021 (no consulting fee received) and also attended a virtual advisory board for Merck for Global Cervical and Ovarian Cancer on 10 July 2023. C.A. has ongoing commitments with AstraZeneca – AZ eVOLVE DMC 26 April 2023; AstraZeneca – Future Clinical Strategies for Endometrial Cancer Virtual Advisory Board 13 March 204 and Verastem – RAMP 301 Steering Committee, 6 March 2024. C.A. participated on a data safety monitoring board and advisory board for Blueprint Medicine – Virtual Advisory Board on 30 June 2021 (no consulting fee received); Merck – Global Cervical and Ovarian Cancer Virtual Advisory Board 10 July 2023; AstraZeneca – AZ eVOLVE DMC 26 April 2023 (ongoing); AstraZeneca – Future Clinical Strategies for Endometrial Cancer Virtual Advisory Board 13 March 2024; Verastem, RAMP 301 Steering Committee, 6 March 2024 (ongoing). C.A. serves on the Board of Directors for both the GOG Foundation and NRG Oncology (unpaid). The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Toon Van Gorp and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ulrike Harjes, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Kaplan-Meier–estimated PFS per RECIST v1.1 as assessed by the investigators in the dMMR population (A) with methylation and (B) with no methylation at interim analysis.
Data cutoff date: December 16, 2022. aBased on a Cox regression model with the Efron method of tie handling with treatment as a covariate. CT, chemotherapy; dMMR, mismatch repair-deficient; HR, hazard ratio; NR, not reached; pembro, pembrolizumab; PFS, progression-free survival; pMMR, mismatch repair-proficient; RECIST, Response Evaluation Criteria in Solid Tumors.
Extended Data Fig. 2 Kaplan-Meier–estimated PFS per RECIST v1.1 as assessed by BICR in the (A) pMMR and (B) dMMR populations at interim analysis.
Data cutoff dates: December 6, 2022 (pMMR); December 16, 2022 (dMMR). aBased on a Cox regression model with the Efron method of tie handling with treatment as a covariate stratified by prior chemotherapy. bOne-sided P value based on log-rank test stratified by prior chemotherapy. CT, chemotherapy; dMMR, mismatch repair-deficient; HR, hazard ratio; NR, not reached; pembro, pembrolizumab; PFS, progression-free survival; pMMR, mismatch repair-proficient; RECIST, Response Evaluation Criteria in Solid Tumors.
Extended Data Fig. 3 Patient disposition in the pMMR and dMMR populations at ad hoc analysis.
Data cutoff date: August 18, 2023. aBy central or local immunohistochemistry. bIncludes alternative therapy (in absence of progression), off treatment due to other complicating disease, symptomatic deterioration, and other (not otherwise specified). AE, adverse event; dMMR, mismatch repair-deficient; ITT, intention to treat; PD, progressive disease; pMMR, mismatch repair-proficient.
Extended Data Fig. 4 Kaplan-Meier–estimated OS in the (A) pMMR and (B) dMMR populations and (C) sensitivity analysis in the pMMR population at ad hoc analysis.
OS data were immature (46.4% information fraction for pMMR population [169/364 events needed for final analysis had occurred] and 29.3% information fraction for dMMR population [44/150 events needed for final analysis had occurred]). Data cutoff date: August 18, 2023. aFollow-up duration is the time from randomization to the date of death or the database cutoff date if the participant is still alive; follow-up duration was not calculated for the sensitivity analysis (panel C) owing to the nature of the bootstrap analysis. bBased on a Cox regression model with the Efron method of tie handling with treatment as a covariate stratified by prior chemotherapy. cOne-sided P value based on log-rank test stratified by prior chemotherapy. CT, chemotherapy; dMMR, mismatch repair-deficient; HR, hazard ratio; NR, not reached; OS, overall survival; pembro, pembrolizumab; pMMR, mismatch repair-proficient.
Extended Data Fig. 5 Investigator-assessed response rates per RECIST v1.1 at ad hoc analysis in the (A) pMMR and (B) dMMR populations.
Analysis included patients in the intention-to-treat population with measurable disease at baseline. Data cutoff date: August 18, 2023. Data shown are the mean ORR and 95% CIs based on the binomial exact method. CR, complete response; CT, chemotherapy; dMMR, mismatch repair-deficient; ORR, objective response rate; pMMR, mismatch repair-proficient; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors.
Extended Data Fig. 6 Kaplan-Meier–estimated DOR per RECIST v1.1 as assessed by the investigators in the (A) pMMR and (B) dMMR populations at ad hoc analysis.
Data cutoff date: August 18, 2023. +, no progressive disease at last disease assessment; CT, chemotherapy; dMMR, mismatch repair-deficient; DOR, duration of response; NR, not reached; pembro, pembrolizumab; pMMR, mismatch repair-proficient; RECIST, Response Evaluation Criteria in Solid Tumors.
Extended Data Fig. 7 Investigator-assessed best percentage change from baseline in target lesions per RECIST v1.1 in the (A) pMMR and (B) dMMR populations at ad hoc analysis.
Data cutoff date: August 18, 2023. CT, chemotherapy; dMMR, mismatch repair-deficient; pembro, pembrolizumab; pMMR, mismatch repair-proficient; RECIST, Response Evaluation Criteria in Solid Tumors.
Supplementary information
Supplementary Information
List of investigators and study sites, methodology (assessments, statistical methods for the confounding effect of subsequent anticancer therapy on OS) and Supplementary Tables 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eskander, R.N., Sill, M.W., Beffa, L. et al. Pembrolizumab plus chemotherapy in advanced or recurrent endometrial cancer: overall survival and exploratory analyses of the NRG GY018 phase 3 randomized trial. Nat Med 31, 1539–1546 (2025). https://doi.org/10.1038/s41591-025-03566-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03566-1
This article is cited by
-
Combination of immunotherapy and chemotherapy as first-line treatment for advanced or recurrent endometrial cancer: a meta-analysis of phase 3 trials
BMC Cancer (2025)
-
Immune checkpoint inhibitors for the treatment of solid tumors and lymphoma in the past 26 years (2000–2025)
Journal of Hematology & Oncology (2025)
-
Molecular subtyping of endometrial carcinoma cell lines uncovers subtype-specific targetable vulnerabilities
npj Precision Oncology (2025)
-
Therapeutic targeting of mismatch repair-deficient cancers
Nature Reviews Clinical Oncology (2025)
-
Dynamic remodeling of tertiary lymphoid structures in response to cancer therapy: a recent review
Cancer Immunology, Immunotherapy (2025)