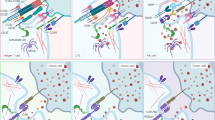
Abstract
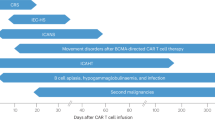
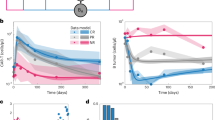
Chimeric antigen receptor (CAR) T cell therapy is associated with a unique spectrum of toxicities that drive morbidity, mortality and patient quality of life. Previous efforts yielded consensus grading systems for the prototypical immunotoxicities—namely, cytokine-release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). These grading systems set the stage for severity-based and standardized treatment protocols that have contributed to a reduction in the acute toxicity burden of CAR T cell therapy and have enabled outpatient administration. However, understanding of CAR T cell therapy has since grown to encompass new targets, new diseases and broader patient populations—including long-term survivors. As side effects are better defined and novel toxicities emerge, there is a need to understand their mechanisms and standardize reporting to improve clinical management. Here we review the current state of knowledge for mortality-defining and rare toxicities of CAR T cell therapies, beyond CRS and ICANS. We discuss mechanisms, including on-target injury, cytokine-associated inflammation and dysregulated recovery, and how these mechanisms affect the timing and management of toxicities. Finally, we define key unmet needs and delineate future priorities and research directions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Locke, F. L. et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 386, 640–654 (2022).
Shah, B. D. et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 398, 491–502 (2021).
Locke, F. L. et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 20, 31–42 (2019).
Wang, M. et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 382, 1331–1342 (2020).
Muller, F. et al. CD19 CAR T-cell therapy in autoimmune disease - a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024).
Schett, G., Mackensen, A. & Mougiakakos, D. CAR T-cell therapy in autoimmune diseases. Lancet 402, 2034–2044 (2023).
Shimabukuro-Vornhagen, A. et al. Cytokine release syndrome. J. Immunother. Cancer 6, 56 (2018).
Karschnia, P. et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood 133, 2212–2221 (2019).
Karschnia, P. et al. Neurologic toxicities following adoptive immunotherapy with BCMA-directed CAR T cells. Blood 142, 1243–1248 (2023).
Wudhikarn, K. et al. DLBCL patients treated with CD19 CAR T cells experience a high burden of organ toxicities but low nonrelapse mortality. Blood Adv. 4, 3024–3033 (2020).
Jain, T. et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 4, 3776–3787 (2020).
Rejeski, K. et al. The CAR-HEMATOTOX score as a prognostic model of toxicity and response in patients receiving BCMA-directed CAR-T for relapsed/refractory multiple myeloma. J. Hematol. Oncol. 16, 88 (2023).
Rejeski, K. et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood 138, 2499–2513 (2021).
Rejeski, K. et al. An international survey on grading, diagnosis, and management of immune effector cell-associated hematotoxicity (ICAHT) following CAR T-cell therapy on behalf of the EBMT and EHA. Hemasphere 7, e889 (2023).
Rejeski, K. et al. Immune effector cell-associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood 142, 865–877 (2023).
Rejeski, K., Jain, M. D., Shah, N. N., Perales, M. A. & Subklewe, M. Immune effector cell-associated haematotoxicity after CAR T-cell therapy: from mechanism to management. Lancet Haematol. 11, e459–e470 (2024).
Hill, J. A. et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 131, 121–130 (2018).
Rejeski, K. et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J. Immunother. Cancer 10, e004475 (2022).
Gudiol, C., Lewis, R. E., Strati, P. & Kontoyiannis, D. P. Chimeric antigen receptor T-cell therapy for the treatment of lymphoid malignancies: is there an excess risk for infection? Lancet Haematol. 8, e216–e228 (2021).
Kampouri, E. et al. Infections after chimeric antigen receptor (CAR)-T-cell therapy for hematologic malignancies. Transpl. Infect. Dis. e14157 (2023).
Wudhikarn, K. et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood Cancer J. 10, 79 (2020).
Hill, J. A. & Seo, S. K. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood 136, 925–935 (2020).
Diorio, C. et al. Quadriparesis and paraparesis following chimeric antigen receptor T-cell therapy in children and adolescents. Blood 144, 1387–1398 (2024).
Hines, M. R. et al. Immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome. Transpl. Cell Ther. 29, 431–438 (2023).
Jain, M. D., Smith, M. & Shah, N. N. How I treat refractory CRS and ICANS after CAR T-cell therapy. Blood 141, 2430–2442 (2023).
Cordas Dos Santos, D. M. et al. A systematic review and meta-analysis of nonrelapse mortality after CAR T cell therapy. Nat. Med. 30, 2667–2678 (2024).
Jain, M. D. et al. Five-year follow-up of standard-of-care axicabtagene ciloleucel for large B-cell lymphoma: results from the US Lymphoma CAR T Consortium. J. Clin. Oncol. 42, 3581–3592 (2024).
Lemoine, J. et al. Nonrelapse mortality after CAR T-cell therapy for large B-cell lymphoma: a LYSA study from the DESCAR-T registry. Blood Adv. 7, 6589–6598 (2023).
DeVita, V. T. Jr. & Chu, E. A history of cancer chemotherapy. Cancer Res. 68, 8643–8653 (2008).
Westin, J. R. et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N. Engl. J. Med. 389, 148–157 (2023).
Harrison, S. J. et al. CAR+ T-cell lymphoma post ciltacabtagene autoleucel therapy for relapsed refractory multiple myeloma. Blood 142, 6939 (2023).
Banerjee, R. et al. Answering the “Doctor, can CAR-T therapy cause cancer?” question in clinic. Blood Adv. 8, 895–898 (2024).
Verdun, N. & Marks, P. Secondary cancers after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 390, 584–586 (2024).
Levine, B. L. et al. Unanswered questions following reports of secondary malignancies after CAR-T cell therapy. Nat. Med. 30, 338–341 (2024).
Tix, T. et al. Second primary malignancies after CAR T-cell therapy: a systematic review and meta-analysis of 5,517 lymphoma and myeloma patients. Clin. Cancer Res. 30, 4690–4700 (2024).
Ghilardi, G. et al. T cell lymphoma and secondary primary malignancy risk after commercial CAR T cell therapy. Nat. Med. 30, 984–989 (2024).
Storgard, R., Rejeski, K., Perales, M. A., Goldman, A. & Shouval, R. T-cell malignant neoplasms after chimeric antigen receptor T-cell therapy. JAMA Oncol. 10, 826–828 (2024).
Chihara, D., Dores, G. M., Flowers, C. R. & Morton, L. M. The bidirectional increased risk of B-cell lymphoma and T-cell lymphoma. Blood 138, 785–789 (2021).
Cordeiro, A. et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol. Blood Marrow Transpl. 26, 26–33 (2020).
Zhao, A. et al. Secondary myeloid neoplasms after CD19 CAR T therapy in patients with refractory/relapsed B-cell lymphoma: case series and review of literature. Front. Immunol. 13, 1063986 (2022).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Neelapu, S. S. et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 141, 2307–2315 (2023).
Kuhnl, A. et al. Early FDG-PET response predicts CAR-T failure in large B-cell lymphoma. Blood Adv. 6, 321–326 (2022).
Wesson, W. et al. Timing of toxicities and non-relapse mortality following CAR T therapy in myeloma. Transpl. Cell Ther. 30, 876–884 (2024).
Ahmed, N. et al. Optimizing the post-CAR T monitoring period in recipients of axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel. Blood Adv. 8, 5346–5354 (2024).
Ventin, M. et al. Implications of high tumor burden on chimeric antigen receptor T-cell immunotherapy: a review. JAMA Oncol. 10, 115–121 (2024).
Jain, M. D. et al. Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma. Blood 137, 2621–2633 (2021).
Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Parker, K. R. et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142 (2020).
Lichtenstein, D. A. et al. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells. Blood 138, 2469–2484 (2021).
Frank, M. J. et al. CD22-directed CAR T-cell therapy for large B-cell lymphomas progressing after CD19-directed CAR T-cell therapy: a dose-finding phase 1 study. Lancet 404, 353–363 (2024).
Sidana, S. et al. Safety and efficacy of standard-of-care ciltacabtagene autoleucel for relapsed/refractory multiple myeloma. Blood 145, 85–97 (2025).
Cohen, A. D. et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 12, 32 (2022).
San-Miguel, J. et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N. Engl. J. Med. 389, 335–347 (2023).
Kumar, A. D. et al. Delayed neurotoxicity after CAR-T in multiple myeloma: results from a Global IMWG Registry. Blood 144, 4758–4758 (2024).
Van De Donk, N. W. C. J. et al. Clinical experience with cranial nerve impairment in the CARTITUDE-1, CARTITUDE-2 cohorts A, B, and C, and CARTITUDE-4 studies of ciltacabtagene autoleucel (Cilta-cel). Blood 142, 3501–3501 (2023).
Kathari, Y. K. et al. Immune-mediated facial nerve paralysis in a myeloma patient post B-cell maturation antigen-targeted chimeric antigen receptor T cells. Haematologica 109, 682–688 (2024).
Graham, C. E. et al. Chemotherapy-induced reversal of ciltacabtagene autoleucel-associated movement and neurocognitive toxicity. Blood 142, 1248–1252 (2023).
Fortuna, G. G. et al. Immune effector cell-associated enterocolitis following chimeric antigen receptor T-cell therapy in multiple myeloma. Blood Cancer J. 14, 180 (2024).
Jahnsen, F. L., Bækkevold, E. S., Hov, J. R. & Landsverk, O. J. Do long-lived plasma cells maintain a healthy microbiota in the gut? Trends Immunol. 39, 196–208 (2018).
Merz, M. et al. Idecabtagene vicleucel or ciltacabtagene autoleucel for relapsed or refractory multiple myeloma: an international multicenter study. Hemasphere 9, e70070 (2025).
Hansen, D. K. et al. Comparison of standard-of-care idecabtagene vicleucel and ciltacabtagene autoleucel in relapsed/refractory multiple myeloma. J. Clin. Oncol. 43, 1597–1609 (2025).
Mailankody, S. et al. GPRC5D-Targeted CAR T cells for myeloma. N. Engl. J. Med. 387, 1196–1206 (2022).
Neri, P. et al. Just scratching the surface: novel treatment approaches for multiple myeloma targeting cell membrane proteins. Nat. Rev. Clin. Oncol. 21, 590–609 (2024).
Rodriguez-Otero, P. et al. GPRC5D as a novel target for the treatment of multiple myeloma: a narrative review. Blood Cancer J. 14, 24 (2024).
Bal, S. et al. Efficacy and safety with extended follow-up in a phase 1 study of BMS-986393, a G-protein-coupled receptor class C group 5 member D (GPRC5D)-targeted CAR T cell therapy, in patients (pts) with heavily pretreated relapsed/refractory (RR) multiple myeloma (MM). Blood 144, 922 (2024).
Perthus, A. et al. Remission after CAR T-cell therapy: do lymphoma patients recover a normal life? Hemasphere 8, e72 (2024).
Johnson, P. C. et al. Longitudinal patient-reported outcomes in patients receiving chimeric antigen receptor T-cell therapy. Blood Adv. 7, 3541–3550 (2023).
Logue, J. M. et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica 106, 978–986 (2021).
Jain, T., Olson, T. S. & Locke, F. L. How I treat cytopenias after CAR T-cell therapy. Blood 141, 2460–2469 (2023).
Vic, S. et al. Transfusion needs after CAR T-cell therapy for large B-cell lymphoma: predictive factors and outcome (a DESCAR-T study). Blood Adv. 8, 1573–1585 (2024).
Rejeski, K., Subklewe, M. & Locke, F. L. Recognizing, defining, and managing CAR-T hematologic toxicities. Hematol. Am. Soc. Hematol. Educ. Program 2023, 198–208 (2023).
Johnsrud, A. et al. Incidence and risk factors associated with bleeding and thrombosis following chimeric antigen receptor T-cell therapy. Blood Adv. 5, 4465–4475 (2021).
Rejeski K. et al. T-ICAHT: grading and prognostic impact of thrombocytopenia after CAR T-cell therapy. Blood https://doi.org/10.1182/blood.2025028833 (2025).
Fried, S. et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 54, 1643–1650 (2019).
Rejeski, K. et al. The CAR-HEMATOTOX score identifies patients at high risk for hematological toxicity, infectious complications, and poor treatment outcomes following brexucabtagene autoleucel for relapsed or refractory MCL. Am. J. Hematol. 98, 1699–1710 (2023).
Rejeski, K. et al. Severe Candida glabrata pancolitis and fatal Aspergillus fumigatus pulmonary infection in the setting of bone marrow aplasia after CD19-directed CAR T-cell therapy - a case report. BMC Infect. Dis. 21, 121 (2021).
Rejeski, K. et al. Severe hematotoxicity after CD19 CAR-T therapy is associated with suppressive immune dysregulation and limited CAR-T expansion. Sci. Adv. 9, eadg3919 (2023).
Cohen, A. D. et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J. Clin. Invest. 129, 2210–2221 (2019).
Rejeski, K. et al. Identifying early infections in the setting of CRS with routine and exploratory serum proteomics and the HT10 score following CD19 CAR-T for relapsed/refractory B-NHL. Hemasphere 7, e858 (2023).
Nair, M. S. et al. Development of ALL-Hematotox: predicting post-CAR T-cell hematotoxicity in B-cell acute lymphoblastic leukemia. Blood 145, 1136–1148 (2025).
Taplitz, R. A. et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 36, 3043–3054 (2018).
Rejeski, K. et al. Applying the EHA/EBMT grading for ICAHT after CAR-T: comparative incidence and association with infections and mortality. Blood Adv. 8, 1857–1868 (2024).
Lievin, R. et al. Effect of early granulocyte-colony-stimulating factor administration in the prevention of febrile neutropenia and impact on toxicity and efficacy of anti-CD19 CAR-T in patients with relapsed/refractory B-cell lymphoma. Bone Marrow Transplant. 57, 431–439 (2022).
Miller, K. C. et al. Effect of granulocyte colony-stimulating factor on toxicities after CAR T cell therapy for lymphoma and myeloma. Blood Cancer J. 12, 146 (2022).
Rejeski, K. et al. Safety and feasibility of stem cell boost as a salvage therapy for severe hematotoxicity after CD19 CAR T-cell therapy. Blood Adv. 6, 4719–4725 (2022).
Gagelmann, N. et al. Hematopoietic stem cell boost for persistent neutropenia after CAR T-cell therapy: a GLA/DRST study. Blood Adv. 7, 555–559 (2023).
Mohan, M. et al. Autologous stem cell boost improves persistent immune effector cell associated hematotoxicity following BCMA directed chimeric antigen receptor T (CAR T) cell therapy in multiple myeloma. Bone Marrow Transplant. 59, 647–652 (2024).
Drillet, G., Lhomme, F., De Guibert, S., Manson, G. & Houot, R. Prolonged thrombocytopenia after CAR T-cell therapy: the role of thrombopoietin receptor agonists. Blood Adv. 7, 537–540 (2023).
Wesson, W. et al. Safety and efficacy of eltrombopag in patients with post-CAR T cytopenias. Eur. J. Haematol. 112, 538–546 (2024).
Lickefett, B. et al. Lymphodepletion - an essential but undervalued part of the chimeric antigen receptor T-cell therapy cycle. Front. Immunol. 14, 1303935 (2023).
Strati, P. et al. Prolonged cytopenia following CD19 CAR T cell therapy is linked with bone marrow infiltration of clonally expanded IFNγ-expressing CD8 T cells. Cell Rep. Med. 4, 101158 (2023).
Kampouri, E., Walti, C. S., Gauthier, J. & Hill, J. A. Managing hypogammaglobulinemia in patients treated with CAR-T-cell therapy: key points for clinicians. Expert Rev. Hematol. 15, 305–320 (2022).
Haidar, G. et al. Invasive mold infections after chimeric antigen receptor-modified T-cell therapy: a case series, review of the literature, and implications for prophylaxis. Clin. Infect. Dis. 71, 672–676 (2020).
Vora, S. B. et al. Infectious complications following CD19 chimeric antigen receptor T-cell therapy for children, adolescents, and young adults. Open Forum Infect. Dis. 7, ofaa121 (2020).
Baird, J. H. et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 5, 143–155 (2021).
Wittmann Dayagi, T. et al. Characteristics and risk factors of infections following CD28-based CD19 CAR-T cells. Leuk. Lymphoma 62, 1692–1701 (2021).
Mikkilineni, L. et al. Infectious complications of CAR T-cell therapy across novel antigen targets in the first 30 days. Blood Adv. 5, 5312–5322 (2021).
Little, J. S. et al. Low incidence of invasive fungal disease following CD19 chimeric antigen receptor T-cell therapy for non-Hodgkin lymphoma. Blood Adv. 6, 4821–4830 (2022).
Kambhampati, S. et al. Infectious complications in patients with relapsed refractory multiple myeloma after BCMA CAR T-cell therapy. Blood Adv. 6, 2045–2054 (2022).
Mohan, M. et al. Risk of infections with B-cell maturation antigen-directed immunotherapy in multiple myeloma. Blood Adv. 6, 2466–2470 (2022).
Josyula, S. et al. Pathogen-specific humoral immunity and infections in B cell maturation antigen-directed chimeric antigen receptor T cell therapy recipients with multiple myeloma. Transpl. Cell Ther. 28, 304 e1–304.e9 (2022).
Wang, Y. et al. Humoral immune reconstitution after anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. Blood Adv. 5, 5290–5299 (2021).
Logue, J. M. et al. Early cytopenias and infections after standard of care idecabtagene vicleucel in relapsed or refractory multiple myeloma. Blood Adv. 6, 6109–6119 (2022).
Kampouri, E. et al. Cytomegalovirus (CMV) reactivation and CMV-specific cell-mediated immunity after chimeric antigen receptor T-cell therapy. Clin. Infect. Dis. 78, 1022–1032 (2024).
Khawaja, F. et al. Cytomegaloviral infections in recipients of chimeric antigen receptor T-cell therapy: an observational study with focus on oncologic outcomes. Open Forum Infect. Dis. 11, ofae422 (2024).
Lin, R. Y. et al. Incidence and outcomes of cytomegalovirus reactivation after chimeric antigen receptor T-cell therapy. Blood Adv. 8, 3813–3822 (2024).
Kampouri, E. et al. Human herpesvirus 6 reactivation and disease are infrequent in chimeric antigen receptor T-cell therapy recipients. Blood 144, 490–495 (2024).
Strati, P. et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B-cell lymphoma. Haematologica 106, 2667–2672 (2021).
Arnold, D. E. et al. Subcutaneous immunoglobulin replacement following CD19-specific chimeric antigen receptor T-cell therapy for B-cell acute lymphoblastic leukemia in pediatric patients. Pediatr. Blood Cancer 67, e28092 (2020).
Deya-Martinez, A. et al. Kinetics of humoral deficiency in CART19-treated children and young adults with acute lymphoblastic leukaemia. Bone Marrow Transplant. 56, 376–386 (2021).
Levine, J. E. et al. Pooled safety analysis of tisagenlecleucel in children and young adults with B cell acute lymphoblastic leukemia. J. Immunother. Cancer 9, e002287 (2021).
Walti, C. S. et al. Antibodies against vaccine-preventable infections after CAR-T cell therapy for B cell malignancies. JCI Insight 6, e146743 (2021).
O’Connor, B. P. et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199, 91–98 (2004).
Bhoj, V. G. et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 128, 360–370 (2016).
Stock, S. et al. Prognostic significance of immune reconstitution following CD19 CAR T-cell therapy for relapsed/refractory B-cell lymphoma. HemaSphere 9, e70062 (2025).
Angelidakis, G. et al. Humoral immunity and antibody responses against diphtheria, tetanus, and pneumococcus after immune effector cell therapies: a prospective study. Vaccines 12, 1070 (2024).
Reynolds, G., Hall, V. G. & Teh, B. W. Vaccine schedule recommendations and updates for patients with hematologic malignancy post-hematopoietic cell transplant or CAR T-cell therapy. Transpl. Infect. Dis. 25, e14109 (2023).
Walti, C. S. et al. Humoral immunogenicity of the seasonal influenza vaccine before and after CAR-T-cell therapy: a prospective observational study. J. Immunother. Cancer 9, e003428 (2021).
Hill, J. A. et al. SARS-CoV-2 vaccination in the first year after hematopoietic cell transplant or chimeric antigen receptor T-cell therapy: a prospective, multicenter, observational study. Clin. Infect. Dis. 79, 542–554 (2024).
Lee, D. et al. Pneumococcal conjugate vaccine does not induce humoral response when administrated within the six months after CD19 CAR T-cell therapy. Transplant. Cell Ther. 29, 271.e1–277.e9 (2023).
Meir, J., Abid, M. A. & Abid, M. B. State of the CAR-T: risk of infections with chimeric antigen receptor T-cell therapy and determinants of SARS-CoV-2 vaccine responses. Transpl. Cell Ther. 27, 973–987 (2021).
Abid, M. B. The denominator in early phase CAR T-cell trials examining novel target antigens. Lancet 402, 354–356 (2023).
Teh, B. W. et al. Consensus position statement on advancing the standardised reporting of infection events in immunocompromised patients. Lancet Infect. Dis. 24, e59–e68 (2024).
Teh, B. W., Mikulska, M., Mueller, N. J. & Slavin, M. A. Goals to score: the need for a minimum reporting dataset in studies of infection events in immunocompromised patients. Transpl. Infect. Dis. 26, e14154 (2024).
Teh, B. W., Reynolds, G. D., Mikulska, M., Mueller, N. J. & Slavin, M. A. Improving infection reporting in hematology treatment trials. Blood Adv. 8, 5925–5926 (2024).
Jain, T. et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-Hodgkin lymphoma: an expert panel opinion from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 25, 2305–2321 (2019).
McDonald, G. B. et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003-2007 versus 2013-2017 cohorts. Ann. Intern. Med. 172, 229–239 (2020).
Shahid, Z. et al. Best practice considerations by the American Society of Transplant and Cellular Therapy: infection prevention and management after chimeric antigen receptor T cell therapy for hematological malignancies. Transplant. Cell Ther. 30, 955–969 (2024).
Shahid, Z. et al. Defining and grading infections in clinical trials involving hematopoietic cell transplantation: a report from the BMT CTN Infectious Disease Technical Committee. Transplant. Cell Ther. 30, 540.e541–540.e513 (2024).
Patel, S. A., Spiegel, J. Y. & Dahiya, S. Second primary cancer after chimeric antigen receptor-T-cell therapy: a review. JAMA Oncol. 11, 174–181 (2025).
Harrison, S. J. et al. CAR+ T-cell lymphoma after cilta-cel therapy for relapsed or refractory myeloma. N. Engl. J. Med. 392, 677–685 (2025).
Perica, K. et al. CD4+ T-cell lymphoma harboring a chimeric antigen receptor integration in TP53. N. Engl. J. Med. 392, 577–583 (2025).
Hamilton, M. P. et al. Risk of second tumors and T-cell lymphoma after CAR T-cell therapy. N. Engl. J. Med. 390, 2047–2060 (2024).
Dulery, R. et al. T cell malignancies after CAR T cell therapy in the DESCAR-T registry. Nat. Med. 31, 1130–1133 (2025).
Kósa, F. et al. Secondary malignancies and survival of FCR-treated patients with chronic lymphocytic leukemia in Central Europe. Cancer Med. 12, 1961–1971 (2023).
Trab, T. et al. Second primary malignancies in patients with lymphoma in Denmark after high-dose chemotherapy and autologous haematopoietic stem-cell transplantation: a population-based, retrospective cohort study. Lancet Haematol. 10, e838–e848 (2023).
Miret, M. et al. Incidence of second primary malignancies in relapsed/refractory B-cell non-Hodgkin’s lymphoma patients in England. Leuk. Res. 127, 107042 (2023).
Joelsson, J. et al. Incidence and time trends of second primary malignancies after non-Hodgkin lymphoma: a Swedish population-based study. Blood Adv. 6, 2657–2666 (2022).
Lamble, A. J. et al. Risk of T-cell malignancy after CAR T-cell therapy in children, adolescents, and young adults. Blood Adv. 8, 3544–3548 (2024).
White, M. C. et al. Age and cancer risk: a potentially modifiable relationship. Am. J. Prev. Med. 46, S7–S15 (2014).
DePinho, R. A. The age of cancer. Nature 408, 248–254 (2000).
Morton, L. M. et al. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol. 5, 318–325 (2019).
Ghilardi, G. et al. Efficacy and safety of bendamustine for lymphodepletion before lisocabtagene maraleucel. J. Hematol. Oncol. 17, 19 (2024).
Ghilardi, G. et al. Bendamustine lymphodepletion before axicabtagene ciloleucel is safe and associates with reduced inflammatory cytokines. Blood Adv. 8, 653–666 (2024).
Ghilardi, G. et al. Bendamustine is safe and effective for lymphodepletion before tisagenlecleucel in patients with refractory or relapsed large B-cell lymphomas. Ann. Oncol. 33, 916–928 (2022).
Restifo, N. P., Dudley, M. E. & Rosenberg, S. A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Mailankody, S. et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat. Med. 29, 422–429 (2023).
Pegram, H. J. et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119, 4133–4141 (2012).
Tennant, M. D., New, C., Ferreira, L. M. R. & O’Neil, R. T. Efficient T cell adoptive transfer in lymphoreplete hosts mediated by transient activation of Stat5 signaling. Mol. Ther. 31, 2591–2599 (2023).
Locke, F. L. et al. Tocilizumab prophylaxis following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Transpl. Cell Ther. 30, 1065–1079 (2024).
Oluwole, O. O. et al. Long-term outcomes of patients with large B-cell lymphoma treated with axicabtagene ciloleucel and prophylactic corticosteroids. Bone Marrow Transplant. 59, 366–372 (2024).
Strati, P. et al. A phase 1 study of prophylactic anakinra to mitigate ICANS in patients with large B-cell lymphoma. Blood Adv. 7, 6785–6789 (2023).
Park, J. H. et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat. Med. 29, 1710–1717 (2023).
Huarte, E. et al. Itacitinib (INCB039110), a JAK1 inhibitor, reduces cytokines associated with cytokine release syndrome induced by CAR T-cell therapy. Clin. Cancer Res. 26, 6299–6309 (2020).
Larson, R. C. et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature 604, 563–570 (2022).
Frigault, M. J. et al. Itacitinib for the prevention of immune effector cell therapy-associated cytokine release syndrome: results from the phase 2 INCB 39110-211 placebo-controlled randomized cohort. Blood 142, 356 (2023).
Fugger, L., Jensen, L. T. & Rossjohn, J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell 181, 63–80 (2020).
Jain, M. D. & Spiegel, J. Y. Imagining the cell therapist: future CAR T cell monitoring and intervention strategies to improve patient outcomes. EJHaem 3, 46–53 (2022).
Sakemura, R., Can, I., Siegler, E. L. & Kenderian, S. S. In vivo CART cell imaging: paving the way for success in CART cell therapy. Mol. Ther. Oncolytics 20, 625–633 (2021).
Liang, E. C. et al. Development and validation of an automated computational approach to grade immune effector cell-associated hematotoxicity. Bone Marrow Transplant. 59, 910–917 (2024).
Neelapu, S. S. et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat. Med. 28, 735–742 (2022).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Mahdi, J. et al. Tumor inflammation-associated neurotoxicity. Nat. Med. 29, 803–810 (2023).
Alkhateeb, H. B. et al. Therapy-related myeloid neoplasms following chimeric antigen receptor T-cell therapy for non-Hodgkin lymphoma. Blood Cancer J. 12, 113 (2022).
Elsallab, M. et al. Second primary malignancies after commercial car T cell therapy: analysis of FDA Adverse Events Reporting System (FAERS). Blood 143, 2099–2105 (2024).
Goldman, A. et al. Adverse cardiovascular and pulmonary events associated with chimeric antigen receptor T-cell therapy. J. Am. Coll. Cardiol. 78, 1800–1813 (2021).
Gutierrez, C., Neilan, T. G. & Grover, N. S. How I approach optimization of patients at risk of cardiac and pulmonary complications after CAR T-cell therapy. Blood 141, 2452–2459 (2023).
Munir, M., Sayed, A., Addison, D. & Epperla, N. Cardiovascular toxicities associated with novel cellular immune therapies. Blood Adv. 8, 6282–6296 (2024).
Deschenes-Simard, X., Santomasso, B. D. & Dahi, P. B. Clinical features, pathophysiology, and management of acute myelopathy following CAR T-cell therapy. Blood 144, 2083–2094 (2024).
Nair, R. et al. Acute leucoencephalomyelopathy and quadriparesis after CAR T-cell therapy. Haematologica 106, 1504–1506 (2021).
Couturier, A. et al. Parkinson-like neurotoxicity in female patients treated with idecabtagene-vicleucel. Hemasphere 8, e131 (2024).
Bal, S. et al. S193: BMS-986393 (CC-95266), a G protein–coupled receptor class C group 5 member D (GPRC5D)–targeted CAR T-cell therapy for relapsed/refractory multiple myeloma (RRMM): results from a phase 1 study. Hemasphere 7, e9863287 (2023).
Bindal, P. et al. A meta-analysis to assess the risk of bleeding and thrombosis following chimeric antigen receptor T-cell therapy: communication from the ISTH SSC Subcommittee on Hemostasis and Malignancy. J. Thromb. Haemost. 22, 2071–2080 (2024).
Rejeski, K. et al. Influence of adipose tissue distribution, sarcopenia, and nutritional status on clinical outcomes after CD19 CAR T-cell therapy. Cancer Immunol. Res. 11, 707–719 (2023).
Valtis, Y. K. et al. Cancer cachexia and weight loss before CAR T-cell therapy for lymphoma are independently associated with poor outcomes. Blood Adv. 9, 151–161 (2024).
Ligon, J. A. et al. Fertility and CAR T-cells: current practice and future directions. Transpl. Cell Ther. 28, 605.e601–605.e608 (2022).
Acknowledgements
K.R. acknowledges funding from the Else Kröner Forschungskolleg (EKFK) within the Munich Clinician Scientist Program (MCSP). This work was further supported by a grant from the Bruno and Helene Jöster Foundation (to K.R.) and the ‘CAR-T Control’ translational group within the Bavarian Center for Cancer Research (BZKF-TLG-22, to K.R.). M.D.J. is supported by the Mark Foundation and the Bankhead-Coley Cancer Biomedical Research Program.
Author information
Authors and Affiliations
Contributions
Conceptualization: K.R.; Investigation: K.R., J.A.H., S.D. and M.D.J.; Formal analysis and visualization: K.R.; Methodology: K.R., J.A.H., S.D. and M.D.J.; Writing—original draft: K.R., J.A.H., S.D. and M.D.J.; Writing—review and editing: K.R., J.A.H., S.D. and M.D.J. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
K.R.: Kite/Gilead: research funding, consultancy, honoraria and travel support; Novartis: honoraria; BMS/Celgene: consultancy, honoraria; Pierre-Fabre: travel support. J.A.H.: research funding: Allovir, Geovax, Takeda and Gilead; consultancy: Allovir, CSL Behring, Geovax, Karius, Gilead and Moderna. S.D.: research funding: Kite Pharma/Gilead, Novartis and Kyverna Therapeutics; advisory board/consulting: Kite Pharmaceuticals/Gilead Pharma, Bristol Myers Squibb, Incyte, Adaptive Biotechnologies. M.D.J.: Kite/Gilead: consultancy/advisory, research funding; Novartis: consultancy/advisory; Incyte: research funding; Lilly: research funding. None of these competing interests were related to the financing of this study.
Peer review
Peer review information
Nature Medicine thanks Roch Houot, Marco Ruella and Jay Spiegel for their contribution to the peer review of this work. Primary Handling Editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Supplementary Tables 1 and 2
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rejeski, K., Hill, J.A., Dahiya, S. et al. Noncanonical and mortality-defining toxicities of CAR T cell therapy. Nat Med 31, 2132–2146 (2025). https://doi.org/10.1038/s41591-025-03813-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41591-025-03813-5
This article is cited by
-
BCMA-directed mRNA CAR T cell therapy for myasthenia gravis: a randomized, double-blind, placebo-controlled phase 2b trial
Nature Medicine (2026)
-
Beyond the infusion: nursing at the vanguard of cytokine release syndrome rescue in CAR-T cell therapy
Clinical and Translational Oncology (2025)