Abstract
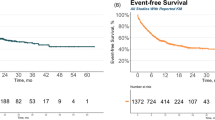
Commercial autologous anti-CD19 chimeric antigen receptor-T cell therapies are effective in B cell malignancies and autoimmune diseases but are limited by personalized manufacturing, high costs and the risk from random chimeric antigen receptor insertion into the genome. To overcome these challenges, we developed YTS109, a hypoimmune allogeneic T cell product engineered using CRISPR–Cas9 to knock out TRAC, PD1, HLA-A, HLA-B and CIITA, with a CD19-targeting synthetic TCR and antigen receptor (STAR) precisely integrated into the TRAC locus to enable physiological, TCR-like signaling. As part of a multi-disease cohort trial, this article includes all enrolled five patients with severe, refractory systemic lupus erythematosus (SLE) complicated by lupus nephritis, who received lymphodepletion followed by YTS109 at 3 × 106 STAR⁺ T cells per kg body weight. Primary endpoints were safety and SLE responder index 4 at month (M) 3. Secondary endpoints included clinical remission and quality-of-life outcomes through to M6. YTS109 was well tolerated, with only mild cytokine release syndrome and no graft-versus-host disease. All five patients in the SLE cohort achieved SLE responder index 4 response at M3, which was sustained through to M6. Four of five patients showed a rapid and sustained reduction in SLE disease activity score (mean 31.30–5.35 by M6), while one patient showed a mild refractory flare-up at M6. Quality-of-life improvements were observed across all four instruments in five patients by 6 months after infusion. Renal biopsies further confirmed resolution of inflammation and tissue restoration. These results demonstrate that YTS109 induced immune resetting and clinical remission, including renal structural restoration, potentially offering a promising therapy for refractory SLE with severe lupus nephritis, pending further validation. ClinicalTrials.gov registration: NCT06379646.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The raw single-cell sequencing data and 10x Visium HD sequencing data reported in this study are available from the Genome Sequence Archive for Human under accession no. HRA011753. The processed single-cell transcriptomic, spatial transcriptomic, mass cytometry and proteomic data reported in this study have been deposited at Zenodo (https://doi.org/10.5281/zenodo.15715577). Individual-level clinical data are available under restricted access because of patient privacy and ethical considerations. Qualified researchers may request access by contacting the corresponding author. Requests will be reviewed by the study’s data access committee to ensure compliance with institutional and regulatory policies. Responses to data access requests can be expected within 2–4 weeks.
Code availability
The code for sequencing processing and data analysis is available at Zenodo (https://doi.org/10.5281/zenodo.15597470).
References
Schett, G. et al. Advancements and challenges in CAR T cell therapy in autoimmune diseases. Nat. Rev. Rheumatol. 20, 531–544 (2024).
Müller, F. et al. CD19 CAR T-cell therapy in autoimmune disease—a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024).
Wang, W. et al. BCMA-CD19 compound CAR T cells for systemic lupus erythematosus: a phase 1 open-label clinical trial. Ann. Rheum. Dis. 83, 1304–1314 (2024).
Wang, X. et al. Allogeneic CD19-targeted CAR-T therapy in patients with severe myositis and systemic sclerosis. Cell 187, 4890–4904 (2024).
Hoi, A., Igel, T., Mok, C. C. & Arnaud, L. Systemic lupus erythematosus. Lancet 403, 2326–2338 (2024).
Liu, Y. et al. Chimeric STAR receptors using TCR machinery mediate robust responses against solid tumors. Sci. Transl. Med. 13, eabb5191 (2021).
Huang, D. et al. TCR-mimicking STAR conveys superior sensitivity over CAR in targeting tumors with low-density neoantigens. Cell Rep. 43, 114949 (2024).
Wang, J. et al. A novel adoptive synthetic TCR and antigen receptor (STAR) T-cell therapy for B-cell acute lymphoblastic leukemia. Am. J. Hematol. 97, 992–1004 (2022).
Aringer, M. et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann. Rheum. Dis. 78, 1151–1159 (2019).
Mikdashi, J. & Nived, O. Measuring disease activity in adults with systemic lupus erythematosus: the challenges of administrative burden and responsiveness to patient concerns in clinical research. Arthritis Res. Ther. 17, 183 (2015).
Petri, M. et al. Combined oral contraceptives in women with systemic lupus erythematosus. N. Engl. J. Med. 353, 2550–2558 (2005).
Isenberg, D. A. et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatol. 44, 902–906 (2005).
Furie, R. A. et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum. 61, 1143–1151 (2009).
Jesus, D. et al. Systemic Lupus Erythematosus Disease Activity Score (SLE-DAS) enables accurate and user-friendly definitions of clinical remission and categories of disease activity. Ann. Rheum. Dis. 80, 1568–1574 (2021).
Gladman, D. D., Ibañez, D. & Urowitz, M. B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 29, 288–291 (2002).
Bajema, I. M. et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 93, 789–796 (2018).
Yee, C.-S. et al. Numerical scoring for the BILAG-2004 index. Rheumatol. 49, 1665–1669 (2010).
Common Terminology Criteria for Adverse Events (CTCAE) v.5.0 (National Cancer Institute, 2017).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Harris, A. C. et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 22, 4–10 (2016).
Saad, A. et al. Hematopoietic cell transplantation, version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl Compr. Canc. Netw. 18, 599–634 (2020).
Franklyn, K. et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann. Rheum. Dis. 75, 1615–1621 (2016).
Bertsias, G. K. et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann. Rheum. Dis. 71, 1771–1782 (2012).
Baker, K. F. et al. Single-cell insights into immune dysregulation in rheumatoid arthritis flare versus drug-free remission. Nat. Commun. 15, 1063 (2024).
Gong, T. & Zhou, R. ApoC3: an ‘alarmin’ triggering sterile inflammation. Nat. Immunol. 21, 9–11 (2020).
Huang, J.-Q. et al. SRPK1/AKT axis promotes oxaliplatin-induced anti-apoptosis via NF-κB activation in colon cancer. J. Transl. Med. 19, 280 (2021).
Zhang, J., Li, S., Liu, F. & Yang, K. Role of CD68 in tumor immunity and prognosis prediction in pan-cancer. Sci. Rep. 12, 7844 (2022).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Ghilardi, G. et al. T cell lymphoma and secondary primary malignancy risk after commercial CAR T cell therapy. Nat. Med. 30, 984–989 (2024).
Qin, C. et al. Single-cell analysis of refractory anti-SRP necrotizing myopathy treated with anti-BCMA CAR-T cell therapy. Proc. Natl Acad. Sci. USA 121, e2315990121 (2024).
Qin, C. et al. Single-cell analysis of anti-BCMA CAR T cell therapy in patients with central nervous system autoimmunity. Sci. Immunol. 9, eadj9730 (2024).
Wu, C. et al. Single-cell transcriptomics reveal potent extrafollicular B cell response linked with granzyme K+ CD8 T cell activation in lupus kidney. Ann Rheum. Dis. 84, 451–466 (2025).
Moroni, G. et al. Lack of EULAR/ERA-EDTA response at 1 year predicts poor long-term renal outcome in patients with lupus nephritis. Ann. Rheum. Dis. 79, 1077–1083 (2020).
Anders, H.-J. et al. Lupus nephritis. Nat. Rev. Dis. Primers 6, 7 (2020).
Rovin, B. H. et al. KDIGO 2024 Clinical Practice Guideline for the management of lupus nephritis. Kidney Int. 105, S1–S69 (2024).
Fanouriakis, A. et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. 83, 15–29 (2024).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Fan, H. C., Fu, G. K. & Fodor, S. P. Expression profiling. Combinatorial labeling of single cells for gene expression cytometry. Science 347, 1258367 (2015).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst. 8, 281–291 (2019).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Traag, V. A., Waltman, L. & van Eck, N. J. From Louvain to Leiden: guaranteeing well-connected communities. Sci. Rep. 9, 5233 (2019).
Sturm, G. et al. Scirpy: a Scanpy extension for analyzing single-cell T-cell receptor-sequencing data. Bioinformatics 36, 4817–4818 (2020).
Stewart, B. J. et al. Spatiotemporal immune zonation of the human kidney. Science 365, 1461–1466 (2019).
Kleshchevnikov, V. et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat. Biotechnol. 40, 661–671 (2022).
Zunder, E. R. et al. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat. Protoc. 10, 316–333 (2015).
Finck, R. et al. Normalization of mass cytometry data with bead standards. Cytometry A 83, 483–494 (2013).
Fang, Z., Liu, X. & Peltz, G. GSEApy: a comprehensive package for performing gene set enrichment analysis in Python. Bioinformatics 39, btac757 (2023).
Acknowledgements
This study was partially supported by BriSTAR Immunotech. H.X. is supported by the Natural Science Foundation of China (grant no. 82320108010) and the Shanghai Collaborative Innovation Cluster Program (grant no. 2024CXJQ01). X. Lin was supported by the National Natural Science Foundation of China (grant nos. 82293664, 82341212 and 31930039) and the Annual Fund from the Tsinghua-Peking Center for Life Sciences. X. Wang is supported by the Project of the National Key R&D Program of China (grant nos. 2024YFC2510306 and 2024YFC2510300), the Natural Science Foundation of China (grant no. 82271816) and the Clinical Research Project of the Shanghai Municipal Health Commission (grant no. 202440161). X. Wu is supported by the Natural Science Foundation of China (grant no. 82271852), the National Key R&D Program of China (grant no. 2022YFF1203100) and the Innovative Clinical Research Project of Shanghai Changzheng Hospital (grant no. 2020YCXYJ-QN04).
Author information
Authors and Affiliations
Contributions
H.X. and X. Lin designed and supervised the study, and reviewed and edited the paper. X. Wang conducted the study and wrote the original draft. Y.Z. acquired the data and performed the clinical evaluation. L.S. and H.Z. supervised the study and edited the paper. Y.H. provided consultation and contributed to the review and editing of the paper. S.Z. and X.Z. monitored the clinical trial. H.W. and R.S. designed the STAR-T cell construct and Jiasheng Wang participated in the in vitro experiments. Y. Li and Z.W. were responsible for manufacturing and the quality control of the cell product. C.H., S.L., X. Liu and X. Wu managed the clinical care of patients. J.L., H.H., L.L., H.L., T.L., L.Z., L.Y., J.Z., Y. Lin, B.N., Z.Z., Xiaofeng Tang, J.D. and Z.M. assisted with the clinical evaluations. K.P., Jiazheng Wang and W.C. performed the bioinformatics analysis. Y.C., S.Y. and R.Y. collected and visualized the data. T.G. managed the nursing care. Xiaojing Tang and B.D. performed the renal biopsies. Y. Liu, N.T. and J. Wu conducted the renal pathology studies. All authors approved the final paper and vouched for the accuracy and completeness of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks George Tsokos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Potential off-target cleavage sites identified by GUIDE-Seq and assessment of potential GVHD risk for YTS109 in NCG mice model.
a, The cleavage sites sequences and dsODN incorporation sites of TRAC, HLA-A/HLA-B, CIITA and PDCD-1 were detected by GUIDE-Seq, annotated as either on-target or off-target, the total number of unique alignments associated with the site, and the identifier Gene ID. b, NCG mice received 1 × 107 Mock-T or YTS109 cells (N = 7 animals per group). T cell expansion was monitored via blood collection from the tail vein at indicated timepoints. Body weight and survival rate were recorded. Data are presented as mean values +/− SEM. Statistical significance was determined with one-way ANOVA (left), two-tailed, unpaired two-sample t test (middle) or Log-rank (Mantel-Cox) test (right). ***p < 0.001; **p < 0.01; *p < 0.05.
Extended Data Fig. 2 Spatial transcriptomic profiling of renal biopsy revealed reduced immune activation and inflammation in the kidney after YTS109 treatment.
a, Multicolor immunofluorescence staining highlighting the distribution and alterations of CD4 (orange) cells. b, Spatial cell type distribution and clustering. HE staining (top); cell type annotation (middle); louvain clustering (bottom). Spatial transcriptomic analysis of renal tissue from Patient 1 before (top) and after (bottom) YTS109 treatment. c, Cell type composition of Cluster 1 (Glomerulus) at BL and M9, showing proportions of 29 annotated cell populations including podocytes, glomerular endothelial cells, monocytes, dendritic cells, T cells, epithelial cells, fibroblasts, and others.
Extended Data Fig. 3 Longitudinal single-cell profiling of peripheral immune compartments reveals individual dynamic immune reconstitution after YTS109 treatment.
a, Stacked bar plots showing the distribution and absolute number of peripheral immune cell populations over time across five patients. b, Stacked bar plots showing the distribution and absolute number of peripheral B cell subsets over time across five patients. c, Proportions and absolute numbers of peripheral B cell subsets after YTS109 treatment by Flow cytometry analysis (memory B cells, naïve B cells, and PBs in five patients before and after YTS109 infusion.
Extended Data Fig. 4 Analysis of plasma proteomics following YTS109 infusion.
a, Principal Component Analysis (PCA) plot for plasma. Dots demonstrate individual sample distribution before (Pre, orange) and after treatment (Post, blue). b, Volcano plot comparing the plasma protein expression at M3 and BL. Red indicates up-regulation at M3. c, Heatmap of differentially expressed proteins from plasma samples at BL, M1, M2, and M3. The color scale ranges from low (0.0) to high (1.0). d, GSEA items of the plasma protein at M3 and BL. Red bars represent up-regulated signaling pathways at M3, and blue bars represent down-regulated signaling pathways. P values were calculated by the two-sided Wilcoxon rank-sum test.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Zhang, Y., Wang, H. et al. Allogeneic CD19-targeting T cells for treatment-refractory systemic lupus erythematosus: a phase 1 trial. Nat Med (2025). https://doi.org/10.1038/s41591-025-03899-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41591-025-03899-x