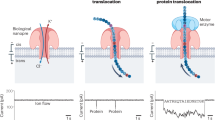
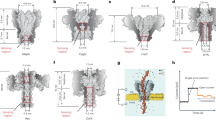
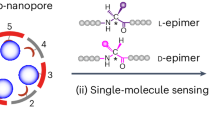
Abstract
The great molecular heterogeneity within single cells demands omics analysis from a single-molecule perspective. Moreover, considering the perpetual metabolism and communication within cells, it is essential to determine the time-series changes of the molecular library, rather than obtaining data at only one time point. Thus, there is an urgent need to develop a single-molecule strategy for this omics analysis to elucidate the biosystem heterogeneity and temporal dynamics. In this Perspective, we explore the potential application of nanopores for single-molecule temporal omics to characterize individual molecules beyond mass, in both a single-molecule and high-throughput manner. Accordingly, recent advances in nanopores available for single-molecule temporal omics are reviewed from the view of single-molecule mass identification, revealing single-molecule heterogeneity and illustrating temporal evolution. Furthermore, we discuss the primary challenges associated with using nanopores for single-molecule temporal omics in complex biological samples, and present the potential strategies and notes to respond to these challenges.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hood, L. A personal view of molecular technology and how it has changed biology. J. Proteome Res. 1, 399–409 (2002).
Oliver, S. G., Winson, M. K., Kell, D. B. & Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 16, 373–378 (1998).
Bosscher, K. D., Desmet, S. J., Clarisse, D., Estébanez-Perpiña, E. & Brunsveld, L. Nuclear receptor crosstalk–defining the mechanisms for therapeutic innovation. Nat. Rev. Endocrinol. 16, 363–377 (2020).
Zhang, Y., Murugesan, P., Huang, K. & Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat. Rev. Cardiol. 17, 170–194 (2020).
Seehausen, O. et al. Genomics and the origin of species. Nat. Rev. Genet. 15, 176–192 (2014).
Wang, Z., Gerstein, M. & Snyder, M. RNA-seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57–63 (2009).
Aebersold, R. & Mann, M. Mass spectrometry-based proteomics. Nature 422, 198–207 (2003).
Newgard, C. B. Metabolomics and metabolic diseases: where do we stand? Cell Metab. 25, 43–56 (2017).
Hasin, Y., Seldin, M. & Lusis, A. Multi-omics approaches to disease. Genome Biol. 18, 83 (2017).
Maitre, L. et al. Multi-omics signatures of the human early life exposome. Nat. Commun. 13, 7024 (2022).
Cairns, R. A., Harris, I. S. & Mak, T. W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 11, 85–95 (2011).
Johnson, C. H., Ivanisevic, J. & Siuzdak, G. Metabolomics: beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 17, 451–459 (2016).
Dueck, H., Eberwine, J. & Kim, J. Variation is function: are single cell differences functionally important? Bioessays 38, 172–180 (2016).
Redit, C., Cha, S. & Ai, N. Single-cell proteomics: challenges and prospects. Nat. Methods 20, 317–318 (2023).
Papalexi, E. & Satija, R. Single-cell RNA sequencing to explore immune cell heterogeneity. Nat. Rev. Immunol. 18, 35–45 (2018).
Lawson, D. A., Kessenbrock, K., Davis, R. T., Pervolarakis, N. & Werb, Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 20, 1349–1360 (2018).
Lewis, S. M. et al. Spatial omics and multiplexed imaging to explore cancer biology. Nat. Methods 18, 997–1012 (2021).
Bressan, D., Battistoni, G. & Hannon, G. J. The dawn of spatial omics. Science 381, eabq4964 (2023).
Sempionatto, J. R., Lasalde-Ramírez, J. A., Mahato, K., Wang, J. & Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 6, 899–915 (2022).
Wu, V. H. et al. Profound phenotypic and epigenetic heterogeneity of the HIV-1-infected CD4+ T cell reservoir. Nat. Immunol. 24, 359–370 (2023).
Ocaranza, M. P. et al. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 17, 116–129 (2019).
Reily, C., Stewart, T. J., Renfrow, M. B. & Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 15, 346–366 (2019).
Wilson, M. R., Satapathy, S. & Vendruscolo, M. Extracellular protein homeostasis in neurodegenerative diseases. Nat. Rev. Neurol. 19, 235–245 (2023).
Manrao, E. A. et al. Reading DNA at single-nucleotide resolution with a mutant MspA nanopore and phi29 DNA polymerase. Nat. Biotechnol. 30, 349–53 (2012). The authors developed a motor protein assistant strategy to experimentally overcome major hurdles to accessing DNA sequencing with nanopore.
Deamer, D., Akeson, M. & Branton, D. Three decades of nanopore sequencing. Nat. Biotechnol. 34, 518–524 (2016).
Cao, C. et al. Discrimination of oligonucleotides of different lengths with a wild-type aerolysin nanopore. Nat. Nanotechnol. 11, 713–718 (2016).
Sutherland, T. C. et al. Structure of peptides investigated by nanopore analysis. Nano Lett. 4, 1273–1277 (2004).
Brinkerhoff, H., Kang, A. S. W., Liu, J., Aksimentiev, A. & Dekker, C. Multiple rereads of single proteins at single–amino acid resolution using nanopores. Science 374, 1509–1513 (2021).
Steffensen, M. B., Rotem, D. & Bayley, H. Single-molecule analysis of chirality in a multicomponent reaction network. Nat. Chem. 6, 603–607 (2014).
Qing, Y., Ionescu, S. A., Pulcu, G. S. & Bayley, H. Directional control of a processive molecular hopper. Science 361, 908–912 (2018).
Kowalczyk, S. W. et al. Single-molecule transport across an individual biomimetic nuclear pore complex. Nat. Nanotechnol. 6, 433–438 (2011).
Burns, J. R., Seifert, A., Fertig, N. & Howorka, S. A biomimetic DNA-based channel for the ligand-controlled transport of charged molecular cargo across a biological membrane. Nat. Nanotechnol. 11, 152–156 (2016).
Kasianowicz, J. J., Brandin, E., Branton, D. & Deamer, D. W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl Acad. Sci. USA 93, 13770–13773 (1996).
Robertson, J. W. F. et al. Single-molecule mass spectrometry in solution using a solitary nanopore. Proc. Natl Acad. Sci. USA 104, 8207–8211 (2007). The authors proposed the concept of single-molecule MS by using nanopore.
Wang, K. et al. Unambiguous discrimination of all 20 proteinogenic amino acids and their modifications by nanopore. Nat. Methods 21, 92–101 (2024).
Kang, X., Cheley, S., Guan, X. & Bayley, H. Stochastic detection of enantiomers. J. Am. Chem. Soc. 128, 10684–10685 (2006). This work first illustrated the ability of nanopores to distinguish the enantiomers of chiral molecules at the single-molecule level.
Ensslen, T., Sarthak, K., Aksimentiev, A. & Behrends, J. C. Resolving isomeric posttranslational modifications using a biological nanopore as a sensor of molecular shape. J. Am. Chem. Soc. 144, 16060–16068 (2022). A description of an engineered nanopore for revealing the single-molecule heterogeneity of posttranslational modifications on peptides.
Nova, I. C. et al. Detection of phosphorylation post-translational modifications along single peptides with nanopores. Nat. Biotechnol. 42, 710–714 (2024).
Jiang, J. et al. Protein nanopore reveals the renin–angiotensin system crosstalk with single-amino-acid resolution. Nat. Chem. 15, 578–586 (2023). This article obtained the temporal evolution processes of a series of native peptides at the sub-minute resolution without sampling and disturbing the system.
Ying, Y. L. et al. Nanopore-based technologies beyond DNA sequencing. Nat. Nanotechnol. 17, 1136–1146 (2022).
Thakur, A. K. & Movileanu, L. Real-time measurement of protein–protein interactions at single-molecule resolution using a biological nanopore. Nat. Biotechnol. 37, 96–101 (2018).
Ho, B., Baryshnikova, A. & Brown, G. W. Unification of protein abundance datasets yields a quantitative Saccharomyces cerevisiae proteome. Cell Syst. 6, 192–205 (2018).
Deng, Y., Bai, Z. & Fan, R. Microtechnologies for single-cell and spatial multi-omics. Nat. Rev. Bioeng. 1, 769–784 (2023).
Jeong, H. et al. Functional analysis of structural variants in single cells using Strand-seq. Nat. Biotechnol. 41, 832–844 (2023).
Lu, T., Ang, C. E. & Zhuang, X. Spatially resolved epigenomic profiling of single cells in complex tissues. Cell 185, 4448–4464 (2022).
Rappez, L. et al. SpaceM reveals metabolic states of single cells. Nat. Methods 18, 799–805 (2021).
Bartosovic, M., Kabbe, M. & Castelo-Branco, G. Single-cell CUT&Tag profiles histone modifications and transcription factors in complex tissues. Nat. Biotechnol. 39, 825–835 (2021).
Oehler, S. et al. A DNA-encoded chemical library based on chiral 4-amino-proline enables stereospecific isozyme-selective protein recognition. Nat. Chem. 15, 1431–1443 (2023).
Xie, N. et al. Neoantigens: promising targets for cancer therapy. Signal Transduct. Target. Ther. 8, 9 (2023).
Rosenberger, F. A. et al. Spatial single-cell mass spectrometry defines zonation of the hepatocyte proteome. Nat. Methods 20, 1530–1536 (2023). This work demonstrated the ability of MS technology to identify 1,700 proteins from a single-cell shape on a tissue slice with spatial annotation.
Mergner, J. et al. Mass-spectrometry-based draft of the Arabidopsis proteome. Nature 579, 409–414 (2020).
Grabarics, M. et al. Mass spectrometry-based techniques to elucidate the sugar code. Chem. Rev. 122, 7840–7908 (2022).
Talaga, D. S. & Li, J. Single-molecule protein unfolding in solid state nanopores. J. Am. Chem. Soc. 131, 9287–9297 (2009).
Reiner, J. E., Kasianowicz, J. J., Nablo, B. J. & Robertson, J. W. F. Theory for polymer analysis using nanopore-based single-molecule mass spectrometry. Proc. Natl Acad. Sci. USA 107, 12080–12085 (2010).
Baaken, G. et al. High-resolution size-discrimination of single nonionic synthetic polymers with a highly charged biological nanopore. ACS Nano 9, 6443–6449 (2015).
Balijepalli, A. et al. Quantifying short-lived events in multistate ionic current measurements. ACS Nano 8, 1547–1553 (2014).
Ouldali, H. et al. Electrical recognition of the twenty proteinogenic amino acids using an aerolysin nanopore. Nat. Biotechnol. 38, 176–181 (2020).
Wang, J. et al. Direct quantification of damaged nucleotides in oligonucleotides using an aerolysin single molecule interface. ACS Cent. Sci. 6, 76–82 (2020).
Piguet, F. et al. Identification of single amino acid differences in uniformly charged homopolymeric peptides with aerolysin nanopore. Nat. Commun. 9, 966 (2018).
Xia, B. et al. Mapping the acetylamino and carboxyl groups on glycans by engineered α-hemolysin nanopores. J. Am. Chem. Soc. 145, 18812–18824 (2023).
Yu, L. et al. Unidirectional single-file transport of full-length proteins through a nanopore. Nat. Biotechnol. 41, 1130–1139 (2023).
Nivala, J., Marks, D. B. & Akeson, M. Unfoldase-mediated protein translocation through an α-hemolysin nanopore. Nat. Biotechnol. 31, 247–50 (2013).
Sauciuc, A., Morozzo della Rocca, B., Tadema, M. J., Chinappi, M. & Maglia, G. Translocation of linearized full-length proteins through an engineered nanopore under opposing electrophoretic force. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01954-x (2023). A description of enhanced electroosmotic flow inside nanopores for facilitating the translocation of full-length proteins.
Martin-Baniandres, P. et al. Enzyme-less nanopore detection of post-translational modifications within long polypeptides. Nat. Nanotechnol. 18, 1335–1340 (2023).
Bakshloo, M. A. et al. Nanopore-based protein identification. J. Am. Chem. Soc. 144, 2716–2725 (2022).
Li, M. Y. et al. Unveiling the heterogenous dephosphorylation of DNA using an aerolysin nanopore. ACS Nano 14, 12571–12578 (2020).
Wang, Y. et al. Identification of nucleoside monophosphates and their epigenetic modifications using an engineered nanopore. Nat. Nanotechnol. 17, 976–983 (2022).
Rosen, C. B., Rodriguez-Larrea, D. & Bayley, H. Single-molecule site-specific detection of protein phosphorylation with a nanopore. Nat. Biotechnol. 32, 179–181 (2014).
Huang, G., Voet, A. & Maglia, G. FraC nanopores with adjustable diameter identify the mass of opposite-charge peptides with 44 dalton resolution. Nat. Commun. 10, 835 (2019).
Niu, H. et al. Direct mapping of tyrosine sulfation states in native peptides by nanopore. Nat. Chem. Biol. https://doi.org/10.1038/s41589-024-01734-x (2024).
Tinoco, I. & Gonzalez, R. L. Biological mechanisms, one molecule at a time. Genes Dev. 25, 1205–1231 (2011).
Lu, H. P., Xun, L. & Xie, X. S. Single-molecule enzymatic dynamics. Science 282, 1877–1882 (1998).
Zhou, X. et al. Differentiating enantiomers by directional rotation of ions in a mass spectrometer. Science 383, 612–618 (2024).
Versloot, R. C. A. et al. Seeing the invisibles: detection of peptide enantiomers, diastereomers, and isobaric ring formation in lanthipeptides using nanopores. J. Am. Chem. Soc. 145, 18355–18365 (2023).
Wang, J. et al. Identification of single amino acid chiral and positional isomers using an electrostatically asymmetric nanopore. J. Am. Chem. Soc. 144, 15072–15078 (2022).
Li, M. Y. et al. Revisiting the origin of nanopore current blockage for volume difference sensing at the atomic level. JACS Au 1, 967–976 (2021).
Li, S. et al. T232K/K238Q aerolysin nanopore for mapping adjacent phosphorylation sites of a single Tau peptide. Small Methods 4, 2000014 (2020).
Galenkamp, N. S., Biesemans, A. & Maglia, G. Directional conformer exchange in dihydrofolate reductase revealed by single-molecule nanopore recordings. Nat. Chem. 12, 481–488 (2020).
Grosberg, A. Y. & Rabin, Y. DNA capture into a nanopore: interplay of diffusion and electrohydrodynamics. J. Chem. Phys. 133, 165102 (2010).
Nomidis, S. K., Hooyberghs, J., Maglia, G. & Carlon, E. DNA capture into the ClyA nanopore: diffusion-limited versus reaction-limited processes. J. Phys. Condens. Matter 30, 304001 (2018).
Hu, Z. L., Li, M. Y., Liu, S. C., Ying, Y. L. & Long, Y. T. A lithium-ion-active aerolysin nanopore for effectively trapping long single-stranded DNA. Chem. Sci. 10, 354–358 (2019).
Sheng, Y., Zhou, K., Liu, L. & Wu, H. A nanopore sensing assay resolves cascade reactions in a multienzyme system. Angew. Chem. Int. Ed. Engl. 61, e202200866 (2022).
Niu, H., Li, M. Y., Ying, Y. L. & Long, Y. T. An engineered third electrostatic constriction of aerolysin to manipulate heterogeneously charged peptide transport. Chem. Sci. 13, 2456–2461 (2022).
Clarke, J. et al. Continuous base identification for single-molecule nanopore DNA sequencing. Nat. Nanotechnol. 4, 265–270 (2009). This article used cyclodextrin as a molecular adaptor of nanopores to demonstrate the accurate identification of four nucleotides and discrimination of single-group differences.
Xin, K. et al. 3D blockage mapping for identifying familial point mutations in single amyloid‐β peptides with a nanopore. Angew. Chem. Int. Ed. Engl. 61, e202209970 (2022).
Li, X., Ying, Y. L., Fu, X., Wan, Y. & Long, Y. T. Single‐molecule frequency fingerprint for ion interaction networks in a confined nanopore. Angew. Chem. Int. Ed. Engl. 60, 24582–24587 (2021).
Wei, Z. X. et al. Learning shapelets for improving single-molecule nanopore sensing. Anal. Chem. 91, 10033–10039 (2019).
Ideker, T., Dutkowski, J. & Hood, L. Boosting signal-to-noise in complex biology: prior knowledge is power. Cell 144, 860–863 (2011).
Vandereyken, K., Sifrim, A., Thienpont, B. & Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 24, 494–515 (2023).
Saelens, W., Cannoodt, R., Todorov, H. & Saeys, Y. A comparison of single-cell trajectory inference methods. Nat. Biotechnol. 37, 547–554 (2019).
Lederer, A. R. & Manno, G. L. The emergence and promise of single-cell temporal-omics approaches. Curr. Opin. Biotech. 63, 70–78 (2020).
Chen, W. et al. Live-seq enables temporal transcriptomic recording of single cells. Nature 608, 733–740 (2022). This work obtained the time-resolved transcriptomics from a single living cell.
Nadappuram, B. P. et al. Nanoscale tweezers for single-cell biopsies. Nat. Nanotechnol. 14, 80–88 (2019).
Faller, M., Niederweis, M. & Schulz, G. E. The structure of a mycobacterial outer-membrane channel. Science 303, 1189–1192 (2004).
Goyal, P. et al. Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature 516, 250–253 (2014).
Zhang, M. et al. Real-time detection of 20 amino acids and discrimination of pathologically relevant peptides with functionalized nanopore. Nat. Methods https://doi.org/10.1038/s41592-024-02208-7 (2024).
Lucas, F. L. R. et al. Automated electrical quantification of vitamin B1 in a bodily fluid using an engineered nanopore sensor. Angew. Chem. Int. Ed. Engl. 60, 22849–22855 (2021).
Greive, S. J., Bacri, L., Cressiot, B. & Pelta, J. Identification of conformational variants for bradykinin biomarker peptides from a biofluid using a nanopore and machine learning. ACS Nano 18, 539–550 (2023).
Marcuccio, F. et al. Single-cell nanobiopsy enables multigenerational longitudinal transcriptomics of cancer cells. Sci. Adv. 10, eadl0515 (2024).
Leitao, S. M. et al. Spatially multiplexed single-molecule translocations through a nanopore at controlled speeds. Nat. Nanotechnol. 18, 1078–1084 (2023).
White, R. J. et al. Single ion-channel recordings using glass nanopore membranes. J. Am. Chem. Soc. 129, 11766–11775 (2007).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (22334006 to Y.-T.L., 22304077 to J.J., 22027806 to Y.-T.L. and 22125403 to R.T.).
Author information
Authors and Affiliations
Contributions
M.-Y.L. and J.J. contributed equally to this work. M.-Y.L., J.J. and Y.-T.L. researched data and wrote the manuscript. M.-Y.L., J.-G.L. and Y.-L.Y. designed the figures. M.-Y.L., J.J., H.N., R.T. and Y.-T.L. contributed substantially to the discussion of the content of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Yansheng Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Arunima Singh, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Table 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, MY., Jiang, J., Li, JG. et al. Nanopore approaches for single-molecule temporal omics: promises and challenges. Nat Methods 22, 241–253 (2025). https://doi.org/10.1038/s41592-024-02492-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41592-024-02492-3
This article is cited by
-
Role of the real first interface in regulating ionic signal of nanochannels
Nature Communications (2025)
-
Nanofluidic devices for single-molecule analytical chemistry
Analytical Sciences (2025)