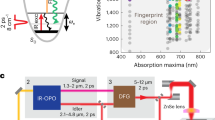
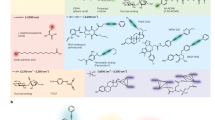
Abstract
Providing molecular fingerprint information, vibrational spectroscopic imaging opens a new window to decipher the function of biomolecules in living systems. While classic vibrational microscopes based on spontaneous Raman scattering or mid-infrared absorption offer rich insights into sample composition, they have very small cross sections or poor spatial resolution. Nonlinear vibrational microscopy, based on coherent Raman scattering or optical photothermal detection of vibrational absorption, overcomes these barriers and enables high-speed and high-sensitivity imaging of chemical bonds in live cells and tissues. Here, we introduce various modalities, including their principles, strengths, weaknesses and data mining methods to the life sciences community. We further provide a guide for prospective users and an outlook on future technological advances.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Duncan, M. D., Reintjes, J. & Manuccia, T. J. Scanning coherent anti-Stokes Raman microscope. Opt. Lett. 7, 350–352 (1982). CARS microscope using a non-collinear beam geometry.
Zumbusch, A., Holtom, G. R. & Xie, X. S. Three-dimensional vibrational imaging by coherent anti-Stokes Raman scattering. Phys. Rev. Lett. 82, 4142–4145 (1999). Collinear CARS microscope. This paper revived the CARS microscopy field.
Cheng, J. X., Volkmer, A., Book, L. D. & Xie, X. S. An epi-detected coherent anti-stokes raman scattering (E-CARS) microscope with high spectral resolution and high sensitivity. J. Phys. Chem. B 105, 1277–1280 (2001). Epi-detected CARS microscope.
Evans, C. L. et al. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proc. Natl Acad. Sci. USA 102, 16807–16812 (2005).
Heuke, S. & Rigneault, H. Coherent Stokes Raman scattering microscopy (CSRS). Nat. Commun. 14, 3337 (2023).
Cheng, J. X., Volkmer, A., Book, L. D. & Xie, X. S. Multiplex coherent anti-stokes Raman scattering microspectroscopy and study of lipid vesicles. J. Phys. Chem. B 106, 8493–8498 (2002).
Wurpel, G. W., Schins, J. M. & Muller, M. Chemical specificity in three-dimensional imaging with multiplex coherent anti-Stokes Raman scattering microscopy. Opt. Lett. 27, 1093–1095 (2002).
Kee, T. W. & Cicerone, M. T. Simple approach to one-laser, broadband coherent anti-Stokes Raman scattering microscopy. Opt. Lett. 29, 2701–2703 (2004).
Camp Jr, C. H. et al. High-speed coherent Raman fingerprint imaging of biological tissues. Nat. Photonics 8, 627–634 (2014). Broadband CARS with high sensitivity.
Volkmer, A., Book, L. D. & Xie, X. S. Time-resolved coherent anti-Stokes Raman scattering microscopy: imaging based on Raman free induction decay. Appl. Phys. Lett. 80, 1505–1507 (2002).
Hellerer, T., Enejder, A. M. K. & Zumbusch, A. Spectral focusing: high spectral resolution spectroscopy with broad-bandwidth laser pulses. Appl. Phys. Lett. 85, 25–27 (2004).
Ogilvie, J. P., Beaurepaire, E., Alexandrou, A. & Joffre, M. Fourier-transform coherent anti-Stokes Raman scattering microscopy. Opt. Lett. 31, 480–482 (2006).
Ideguchi, T. et al. Coherent Raman spectro-imaging with laser frequency combs. Nature 502, 355–358 (2013).
Hashimoto, K., Takahashi, M., Ideguchi, T. & Goda, K. Broadband coherent Raman spectroscopy running at 24,000 spectra per second. Sci. Rep. 6, 21036 (2016).
Kinegawa, R. et al. High-speed broadband Fourier-transform coherent anti-stokes Raman scattering spectral microscopy. J. Raman Spectrosc. 50, 1141–1146 (2019).
Heinrich, C., Bernet, S. & Ritsch-Marte, M. Wide-field coherent anti-Stokes Raman scattering microscopy. Appl. Phys. Lett. 84, 816–818 (2004).
Toytman, I., Cohn, K., Smith, T., Simanovskii, D. & Palanker, D. Wide-field coherent anti-Stokes Raman scattering microscopy with non-phase-matching illumination. Opt. Lett. 32, 1941–1943 (2007).
Heinrich, C., Hofer, A., Bernet, S. & Ritsch-Marte, M. Coherent anti-Stokes Raman scattering microscopy with dynamic speckle illumination. N. J. Phys. 10, 023029 (2008).
Shi, K., Li, H., Xu, Q., Psaltis, D. & Liu, Z. Coherent anti-stokes Raman holography for chemically selective single-shot nonscanning 3D imaging. Phys. Rev. Lett. 104, 093902 (2010).
Lei, M., Winterhalder, M., Selm, R. & Zumbusch, A. Video-rate wide-field coherent anti-Stokes Raman scattering microscopy with collinear nonphase-matching illumination. J. Biomed. Opt. 16, 021102 (2011).
Berto, P., Gachet, D., Bon, P., Monneret, S. & Rigneault, H. Wide-field vibrational phase imaging. Phys. Rev. Lett. 109, 093902 (2012).
Zong, C. et al. Wide-field surface-enhanced coherent anti-Stokes Raman scattering microscopy. ACS Photonics 9, 1042–1049 (2022).
Fantuzzi, E. M. et al. Wide-field coherent anti-Stokes Raman scattering microscopy using random illuminations. Nat. Photonics 17, 1097–1104 (2023).
Ploetz, E., Laimgruber, S., Berner, S., Zinth, W. & Gilch, P. Femtosecond stimulated Raman microscopy. Appl. Phys. B 87, 389–393 (2007). SRS microscope.
Freudiger, C. W. et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008). High-speed SRS microscope achieving high-sensitivity biomedical imaging with chemical specificity.
Nandakumar, P., Kovalev, A. & Volkmer, A. Vibrational imaging based on stimulated Raman scattering microscopy. N. J. Phys. 11, 033026 (2009).
Ozeki, Y., Dake, F., Kajiyama, S. I., Fukui, K. & Itoh, K. Analysis and experimental assessment of the sensitivity of stimulated Raman scattering microscopy. Opt. Express 17, 3651–3658 (2009).
Saar, B. G. et al. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 330, 1368–1370 (2010).
Li, H. et al. Imaging chemical kinetics of radical polymerization with an ultrafast coherent Raman microscope. Adv. Sci. 7, 1903644 (2020).
Andresen, E. R., Berto, P. & Rigneault, H. Stimulated Raman scattering microscopy by spectral focusing and fiber-generated soliton as Stokes pulse. Opt. Lett. 36, 2387–2389 (2011).
Beier, H. T., Noojin, G. D. & Rockwell, B. A. Stimulated Raman scattering using a single femtosecond oscillator with flexibility for imaging and spectral applications. Opt. Express 19, 18885–18892 (2011).
Liao, C.-S. et al. Stimulated Raman spectroscopic imaging by microsecond delay-line tuning. Optica 3, 1377–1380 (2016).
Lin, H. et al. Microsecond fingerprint stimulated Raman spectroscopic imaging by ultrafast tuning and spatial-spectral learning. Nat. Commun. 12, 3052 (2021).
Alshaykh, M. S. et al. High-speed stimulated hyperspectral Raman imaging using rapid acousto-optic delay lines. Opt. Lett. 42, 1548–1551 (2017).
Liao, C. S. et al. Microsecond scale vibrational spectroscopic imaging by multiplex stimulated Raman scattering microscopy. Light Sci. Appl. 4, e265 (2015).
Fu, D. et al. Quantitative chemical imaging with multiplex stimulated Raman scattering microscopy. J. Am. Chem. Soc. 134, 3623–3626 (2012).
Suzuki, Y. et al. Label-free chemical imaging flow cytometry by high-speed multicolor stimulated Raman scattering. Proc. Natl Acad. Sci. USA 116, 15842–15848 (2019).
Karpf, S., Eibl, M., Wieser, W., Klein, T. & Huber, R. A time-encoded technique for fibre-based hyperspectral broadband stimulated Raman microscopy. Nat. Commun. 6, 6784 (2015).
Ozeki, Y. et al. High-speed molecular spectral imaging of tissue with stimulated Raman scattering. Nat. Photonics 6, 844–850 (2012).
Würthwein, T. et al. Multi-color stimulated Raman scattering with a frame-to-frame wavelength-tunable fiber-based light source. Biomed. Opt. Express 12, 6228–6236 (2021).
Wei, L. et al. Super-multiplex vibrational imaging. Nature 544, 465–470 (2017).
Xiong, H. et al. Stimulated Raman excited fluorescence spectroscopy and imaging. Nat. Photonics 13, 412–417 (2019).
Xiong, H. et al. Super-resolution vibrational microscopy by stimulated Raman excited fluorescence. Light Sci. Appl. 10, 87 (2021).
Ao, J. et al. Switchable stimulated Raman scattering microscopy with photochromic vibrational probes. Nat. Commun. 12, 3089 (2021).
Shou, J. et al. Super-resolution vibrational imaging based on photoswitchable Raman probe. Sci. Adv. 9, eade9118 (2023).
Ao, J. et al. Photoswitchable vibrational nanoscopy with sub-100-nm optical resolution. Adv. Photonics 5, 066001 (2023).
Zong, C. et al. Plasmon-enhanced stimulated Raman scattering microscopy with single-molecule detection sensitivity. Nat. Commun. 10, 5318 (2019).
Zhuge, M. et al. Ultrasensitive vibrational imaging of retinoids by visible preresonance stimulated Raman scattering microscopy. Adv. Sci. 8, 2003136 (2021).
Bi, Y. et al. Near-resonance enhanced label-free stimulated Raman scattering microscopy with spatial resolution near 130 nm. Light Sci. Appl. 7, 81 (2018).
Koehl, R. M., Adachi, S. & Nelson, K. A. Real-space polariton wave packet imaging. J. Chem. Phys. 110, 1317–1320 (1999).
Raanan, D. et al. Sub-second hyper-spectral low-frequency vibrational imaging via impulsive Raman excitation. Opt. Lett. 44, 5153–5156 (2019).
Chen, X. et al. Volumetric chemical imaging by stimulated Raman projection microscopy and tomography. Nat. Commun. 8, 15117 (2017).
Wang, W. & Huang, Z. Stimulated Raman scattering tomography for rapid three-dimensional chemical imaging of cells and tissue. Adv. Photonics 6, 026001 (2024).
Robles, F. E., Zhou, K. C., Fischer, M. C. & Warren, W. S. Stimulated Raman scattering spectroscopic optical coherence tomography. Optica 4, 243–246 (2017).
Lin, P. et al. Volumetric chemical imaging in vivo by a remote-focusing stimulated Raman scattering microscope. Opt. Express 28, 30210–30221 (2020).
Nose, K. et al. Sensitivity enhancement of fiber-laser-based stimulated Raman scattering microscopy by collinear balanced detection technique. Opt. Express 20, 13958–13965 (2012).
Freudiger, C. W. et al. Stimulated Raman scattering microscopy with a robust fibre laser source. Nat. Photonics 8, 153–159 (2014).
Crisafi, F. et al. In-line balanced detection stimulated Raman scattering microscopy. Sci. Rep. 7, 10745 (2017).
Orringer, D. A. et al. Rapid intraoperative histology of unprocessed surgical specimens via fibre-laser-based stimulated Raman scattering microscopy. Nat. Biomed. Eng. 1, 0027 (2017). Clinical translation of stimulated Raman histology for intraoperative use.
Liao, C.-S. et al. In vivo and in situ spectroscopic imaging by a handheld stimulated Raman scattering microscope. ACS Photonics 5, 947–954 (2017).
Lin, H. & Cheng, J. X. Computational coherent Raman scattering imaging: breaking physical barriers by fusion of advanced instrumentation and data science. eLight 3, 6 (2023).
Zhang, D. et al. Depth-resolved mid-infrared photothermal imaging of living cells and organisms with submicrometer spatial resolution. Sci. Adv. 2, e1600521 (2016). MIP imaging of living systems. This paper led to mIRage, a commercial product that is worldwide used.
Li, Z., Aleshire, K., Kuno, M. & Hartland, G. V. Super-resolution far-field infrared imaging by photothermal heterodyne imaging. J. Phys. Chem. B 121, 8838–8846 (2017).
Dong, P. T. et al. Polarization-sensitive stimulated Raman scattering imaging resolves amphotericin B orientation in Candida membrane. Sci. Adv. 7, eabd5230 (2021).
Yurdakul, C., Zong, H. N., Bai, Y. R., Cheng, J. X. & Ünlü, M. S. Bond-selective interferometric scattering microscopy. J. Phys. D. 54, 364002 (2021).
Xia, Q. et al. Single virus fingerprinting by widefield interferometric defocus-enhanced mid-infrared photothermal microscopy. Nat. Commun. 14, 6655 (2023).
Yuan, T., Riobo, L., Gasparin, F., Ntziachristos, V. & Pleitez, M. A. Phase-shifting optothermal microscopy enables live-cell mid-infrared hyperspectral imaging of large cell populations at high confluency. Sci. Adv. 10, eadj7944 (2024).
Zhang, D. et al. Bond-selective transient phase imaging via sensing of the infrared photothermal effect. Light Sci. Appl. 8, 116 (2019).
Schnell, M. et al. All-digital histopathology by infrared-optical hybrid microscopy. Proc. Natl Acad. Sci. USA 117, 3388–3396 (2020).
Toda, K., Tamamitsu, M. & Ideguchi, T. Adaptive dynamic range shift (ADRIFT) quantitative phase imaging. Light Sci. Appl. 10, 1 (2021).
Zhang, Y. et al. Fluorescence-detected mid-infrared photothermal microscopy. J. Am. Chem. Soc. 143, 11490–11499 (2021).
Li, M. et al. Fluorescence-detected mid-infrared photothermal microscopy. J. Am. Chem. Soc. 143, 10809–10815 (2021).
Yin, J. et al. Video-rate mid-infrared photothermal imaging by single-pulse photothermal detection per pixel. Sci. Adv. 9, eadg8814 (2023).
He, H. et al. Mapping enzyme activity in living systems by real-time mid-infrared photothermal imaging of nitrile chameleons. Nat. Methods 21, 342–352 (2024).
Adhikari, S. et al. Photothermal microscopy: imaging the optical absorption of single nanoparticles and single molecules. ACS Nano 14, 16414–16445 (2020).
Samolis, P. D. & Sander, M. Y. Phase-sensitive lock-in detection for high-contrast mid-infrared photothermal imaging with sub-diffraction limited resolution. Opt. Express 27, 2643–2655 (2019).
Yin, J. et al. Nanosecond-resolution photothermal dynamic imaging via MHZ digitization and match filtering. Nat. Commun. 12, 7097 (2021).
Samolis, P. D., Zhu, X. & Sander, M. Y. Time-resolved mid-infrared photothermal microscopy for imaging water-embedded axon bundles. Anal. Chem. 95, 16514–16521 (2023).
Bai, Y. et al. Ultrafast chemical imaging by widefield photothermal sensing of infrared absorption. Sci. Adv. 5, eaav7127 (2019).
Toda, K., Tamamitsu, M., Nagashima, Y., Horisaki, R. & Ideguchi, T. Molecular contrast on phase-contrast microscope. Sci. Rep. 9, 9957 (2019).
Ishigane, G. et al. Label-free mid-infrared photothermal live-cell imaging beyond video rate. Light Sci. Appl. 12, 174 (2023).
Tamamitsu, M. et al. Label-free biochemical quantitative phase imaging with mid-infrared photothermal effect. Optica 7, 359–366 (2020).
Zhao, J. et al. Bond-selective intensity diffraction tomography. Nat. Commun. 13, 7767 (2022).
Zhu, Y. et al. Stimulated Raman photothermal microscopy toward ultrasensitive chemical imaging. Sci. Adv. 9, eadi2181 (2023). Stimulated Raman photothermal microscope.
Wilson, R. H., Nadeau, K. P., Jaworski, F. B., Tromberg, B. J. & Durkin, A. J. Review of short-wave infrared spectroscopy and imaging methods for biological tissue characterization. J. Biomed. Opt. 20, 030901 (2015).
Wang, P., Rajian, J. R. & Cheng, J. X. Spectroscopic imaging of deep tissue through photoacoustic detection of molecular vibration. J. Phys. Chem. Lett. 4, 2177–2185 (2013).
Ni, H. et al. Millimetre-deep micrometre-resolution vibrational imaging by shortwave infrared photothermal microscopy. Nat. Photonics 18, 944–951 (2024). Vibrational photothermal microscope in the short-wave infrared window enabling subcellular-resolution and millimeter-deep chemical imaging.
Wang, L. et al. Overtone photothermal microscopy for high-resolution and high-sensitivity vibrational imaging. Nat. Commun. 15, 5374 (2024).
Wang, H. W. et al. Label-free bond-selective imaging by listening to vibrationally excited molecules. Phys. Rev. Lett. 106, 238106 (2011). Bond-selective photoacoustic microscope.
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photonics 7, 205–209 (2013).
Wang, L. V. & Wu, H. I. Biomedical Optics: Principles and Imaging (John Wiley & Sons, 2012).
Sim, J. Y., Ahn, C. G., Jeong, E. J. & Kim, B. K. In vivo microscopic photoacoustic spectroscopy for non-invasive glucose monitoring invulnerable to skin secretion products. Sci. Rep. 8, 1059 (2018).
Pleitez, M. A. et al. Label-free metabolic imaging by mid-infrared optoacoustic microscopy in living cells. Nat. Biotechnol. 38, 293–296 (2020).
Uluc, N. et al. Non-invasive measurements of blood glucose levels by time-gating mid-infrared optoacoustic signals. Nat. Metab. 6, 678–686 (2024).
Shi, J. et al. High-resolution, high-contrast mid-infrared imaging of fresh biological samples with ultraviolet-localized photoacoustic microscopy. Nat. Photonics 13, 609–615 (2019). MIPA microscope.
Xu, Z., Zhu, Q. & Wang, L. V. In vivo photoacoustic tomography of mouse cerebral edema induced by cold injury. J. Biomed. Opt. 16, 066020 (2011).
Jansen, K., van der Steen, A. F., van Beusekom, H. M., Oosterhuis, J. W. & van Soest, G. Intravascular photoacoustic imaging of human coronary atherosclerosis. Opt. Lett. 36, 597–599 (2011).
Wang, P. et al. High-speed intravascular photoacoustic imaging of lipid-laden atherosclerotic plaque enabled by a 2-kHz barium nitrite raman laser. Sci. Rep. 4, 6889 (2014).
Li, R. et al. High-speed intraoperative assessment of breast tumor margins by multimodal ultrasound and photoacoustic tomography. Med. Devices Sens. 1, e10018 (2018).
Yakovlev, V. V. et al. Stimulated Raman photoacoustic imaging. Proc. Natl Acad. Sci. USA 107, 20335–20339 (2010).
Raghunathan, V., Han, Y., Korth, O., Ge, N. H. & Potma, E. O. Rapid vibrational imaging with sum frequency generation microscopy. Opt. Lett. 36, 3891–3893 (2011).
Hanninen, A. M., Prince, R. C., Ramos, R., Plikus, M. V. & Potma, E. O. High-resolution infrared imaging of biological samples with third-order sum-frequency generation microscopy. Biomed. Opt. Express 9, 4807–4817 (2018).
Whaley-Mayda, L., Guha, A., Penwell, S. B. & Tokmakoff, A. Fluorescence-encoded infrared vibrational spectroscopy with single-molecule sensitivity. J. Am. Chem. Soc. 143, 3060–3064 (2021).
Wang, H. et al. Bond-selective fluorescence imaging with single-molecule sensitivity. Nat. Photonics 17, 846–855 (2023).
Yan, C. et al. Multidimensional widefield infrared-encoded spontaneous emission microscopy: distinguishing chromophores by ultrashort infrared pulses. J. Am. Chem. Soc. 146, 1874–1886 (2024).
Vanden-Hehir, S. et al. Alkyne-tagged PLGA allows direct visualization of nanoparticles in vitro and ex vivo by stimulated Raman scattering microscopy. Biomacromolecules 20, 4008–4014 (2019).
Xia, Q. et al. Click-free imaging of carbohydrate trafficking in live cells using an azido photothermal probe. Sci. Adv. 10, eadq0294 (2024).
Tague, N. et al. Longitudinal single-cell imaging of engineered strains with stimulated Raman scattering to characterize heterogeneity in fatty acid production. Adv. Sci. 10, e2206519 (2023).
Ge, X. et al. SRS-FISH: a high-throughput platform linking microbiome metabolism to identity at the single-cell level. Proc. Natl Acad. Sci. USA 119, e2203519119 (2022).
Zhang, J. et al. Visualization of a limonene synthesis metabolon inside living bacteria by hyperspectral SRS microscopy. Adv. Sci. 9, e2203887 (2022).
Wakisaka, Y. et al. Probing the metabolic heterogeneity of live Euglena gracilis with stimulated Raman scattering microscopy. Nat. Microbiol. 1, 16124 (2016).
Schiessl, K. T. et al. Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat. Commun. 10, 762 (2019).
Zhang, M. et al. Rapid determination of antimicrobial susceptibility by stimulated Raman scattering imaging of D2O metabolic incorporation in a single bacterium. Adv. Sci. 7, 2001452 (2020).
Tan, Y., Lin, H. & Cheng, J. X. Profiling single cancer cell metabolism via high-content SRS imaging with chemical sparsity. Sci. Adv. 9, eadg6061 (2023).
Zhao, G. et al. Ovarian cancer cell fate regulation by the dynamics between saturated and unsaturated fatty acids. Proc. Natl Acad. Sci. USA 119, e2203480119 (2022).
Bai, Y. et al. Single-cell mapping of lipid metabolites using an infrared probe in human-derived model systems. Nat. Commun. 15, 350 (2024).
Li, J. et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell 20, 303–314 (2017).
Zhang, X. et al. Label-free live-cell imaging of nucleic acids using stimulated Raman scattering microscopy. Chemphyschem 13, 1054–1059 (2012).
Lu, F. K. et al. Label-free DNA imaging in vivo with stimulated Raman scattering microscopy. Proc. Natl Acad. Sci. USA 112, 11624–11629 (2015).
Oh, S. et al. Protein and lipid mass concentration measurement in tissues by stimulated Raman scattering microscopy. Proc. Natl Acad. Sci. USA 119, e2117938119 (2022).
Lim, J. M. et al. Cytoplasmic protein imaging with mid-infrared photothermal microscopy: cellular dynamics of live neurons and oligodendrocytes. J. Phys. Chem. Lett. 10, 2857–2861 (2019).
Hu, F. et al. Vibrational imaging of glucose uptake activity in live cells and tissues by stimulated Raman scattering. Angew. Chem. Int. Ed. Engl. 54, 9821–9825 (2015).
Li, Y. et al. Microglial lipid droplet accumulation in tauopathy brain is regulated by neuronal AMPK. Cell Metab. 36, 1351–1370 (2024).
Gustavsson, N. et al. Correlative optical photothermal infrared and X-ray fluorescence for chemical imaging of trace elements and relevant molecular structures directly in neurons. Light Sci. Appl. 10, 151 (2021).
Klementieva, O. et al. Super-resolution infrared imaging of polymorphic amyloid aggregates directly in neurons. Adv. Sci. 7, 1903004 (2020). Application of MIP microscopy to protein structure analysis.
Wang, M. C., Min, W., Freudiger, C. W., Ruvkun, G. & Xie, X. S. RNAi screening for fat regulatory genes with SRS microscopy. Nat. Methods 8, 135–138 (2011).
Wang, P. et al. Imaging lipid metabolism in live Caenorhabditis elegans using fingerprint vibrations. Angew. Chem. Int. Ed. Engl. 53, 11787–11792 (2014).
Chen, W. W. et al. Spectroscopic coherent Raman imaging of Caenorhabditis elegans reveals lipid particle diversity. Nat. Chem. Biol. 16, 1087–1095 (2020).
Li, Y., Zhang, W., Fung, A. A. & Shi, L. DO-SRS imaging of diet regulated metabolic activities in Drosophila during aging processes. Aging Cell 21, e13586 (2022).
Hollon, T. C. et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat. Med. 26, 52–58 (2020).
Ji, M. et al. Label-free imaging of amyloid plaques in Alzheimer’s disease with stimulated Raman scattering microscopy. Sci. Adv. 4, eaat7715 (2018).
Randall, E. C. et al. Integrated mapping of pharmacokinetics and pharmacodynamics in a patient-derived xenograft model of glioblastoma. Nat. Commun. 9, 4904 (2018).
Ji, M. et al. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci. Transl. Med. 5, 201ra119 (2013). Use of SRS microscopy for histopathology.
Sethuraman, S., Amirian, J. H., Litovsky, S. H., Smalling, R. W. & Emelianov, S. Y. Ex vivo characterization of atherosclerosis using intravascular photoacoustic imaging. Opt. Express 15, 16657–16666 (2007).
Wu, W., Wang, P., Cheng, J. X. & Xu, X. M. Assessment of white matter loss using bond-selective photoacoustic imaging in a rat model of contusive spinal cord injury. J. Neurotrauma 31, 1998–2002 (2014).
Tian, F. et al. Monitoring peripheral nerve degeneration in ALS by label-free stimulated Raman scattering imaging. Nat. Commun. 7, 13283 (2016).
Wang, B. et al. In vivo intravascular ultrasound-guided photoacoustic imaging of lipid in plaques using an animal model of atherosclerosis. Ultrasound Med. Biol. 38, 2098–2103 (2012).
Li, M. et al. Ultrasensitive in vivo infrared spectroscopic imaging via oblique photothermal microscopy. Preprint at bioRxiv https://doi.org/10.1101/2024.10.02.616360 (2024).
Wei, L. et al. Live-cell imaging of alkyne-tagged small biomolecules by stimulated Raman scattering. Nat. Methods 11, 410–412 (2014).
Shi, L. et al. Highly-multiplexed volumetric mapping with Raman dye imaging and tissue clearing. Nat. Biotechnol. 40, 364–373 (2022).
Kamp, M. et al. Raman micro-spectroscopy reveals the spatial distribution of fumarate in cells and tissues. Nat. Commun. 15, 5386 (2024).
Liu, X., Shi, L., Zhao, Z., Shu, J. & Min, W. VIBRANT: spectral profiling for single-cell drug responses. Nat. Methods 21, 501–511 (2024).
Su, Y. et al. Steam disinfection releases micro(nano)plastics from silicone-rubber baby teats as examined by optical photothermal infrared microspectroscopy. Nat. Nanotechnol. 17, 76–85 (2022).
Wei, M. et al. [Review on vibrational probes.] Nat. Methods https://doi.org/xxx (2025).
Fournier, D., Lepoutre, F. & Boccara, A. C. Tomographic approach for photothermal imaging using the mirage effect. J. Phys. Colloq. 44, C6-479–C476-482 (1983).
Furstenberg, R, Kendziora, C. A., Papantonakis, M. R., Nguyen, V., & McGill, R. A. Chemical imaging using infrared photothermal microspectroscopy. in Proceedings of SPIE Defense, Security, and Sensing 8374, 837411 (2012).
Bai, Y., Yin, J. & Cheng, J. -X. Bond-selective imaging by optically sensing the mid-infrared photothermal effect. Sci. Adv. 7, eabg1559 (2021).
Xia, Q., Yin, J., Guo, Z. & Cheng, J. -X. Mid-infrared photothermal microscopy: principle, instrumentation, applications. J. Phys. Chem. B 126, 8597–8613 (2022).
Product news. Microscope improves infrared spectroscopy. J. Fail. Anal. Prev. 18, 250–251 (2018).
Fu, P. C. et al. Super-resolution imaging of non-fluorescent molecules by photothermal relaxation localization microscopy. Nat. Photonics 17, 330–337 (2023).
Tamamitsu, M. et al. Mid-infrared wide-field nanoscopy. Nat. Photonics 18, 738–743 (2024).
Acknowledgements
We acknowledge grants from the National Institutes of Health (R35 GM136223, R01 EB032391, R01 EB035429, R01 AI141439, R01 CA224275 and R33CA287046) and the Chan Zuckerberg Initiative DAF (2023-321163), an advised fund of Silicon Valley Community Foundation.
Author information
Authors and Affiliations
Contributions
J.-X.C. drafted the outline, introduction, outlook and two boxes. Y.Y. drafted the CARS section. H.N. drafted the SRS and SWIP sections. J.A. drafted the data science section. R.B. and Q.X. drafted the MIP section. X.G. and H.N. drafted the SRP and SWIP section. Q.X., J.A. and Y.Y. drafted the applications and user guide section. J.A., Q.X. and Y.Y. contributed to manuscript revision.
Corresponding author
Ethics declarations
Competing interests
J.-X.C. declares competing interests with VibroniX and Photothermal Spectroscopy, which did not fund this work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Methods thanks Caitlin Davis, Vladislav Yakovlev, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Rita Strack, in collaboration with the Nature Methods team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes and Supplementary Tables 1–5
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, JX., Yuan, Y., Ni, H. et al. Advanced vibrational microscopes for life science. Nat Methods 22, 912–927 (2025). https://doi.org/10.1038/s41592-025-02655-w
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41592-025-02655-w