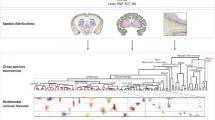
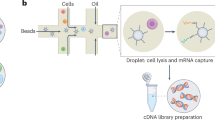
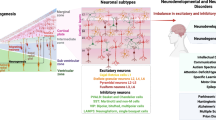
Abstract
Single-cell or single-nucleus transcriptomics is a powerful tool for identifying cell types and cell states. However, hypotheses derived from these assays, including gene expression information, require validation, and their functional relevance needs to be established. The choice of validation depends on numerous factors. Here, we present types of orthogonal and functional validation experiment to strengthen preliminary findings obtained using single-cell and single-nucleus transcriptomics as well as the challenges and limitations of these approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Adameyko, I. et al. Applying single-cell/nucleus genomics to studies of cellular heterogeneity and cell fate transitions in the nervous system. Nat. Neurosci. https://doi.org/10.1038/s41593-024-01827-9 (2024).
Bonev, B. et al. Opportunities and challenges of single-cell and spatially resolved genomics methods for neuroscience discovery. Nat. Neurosci. https://doi.org/10.1038/s41593-024-01806-0 (2024).
Luecken, M. D. & Theis, F. J. Current best practices in single-cell RNA-seq analysis: a tutorial. Mol. Syst. Biol. 15, e8746 (2019).
Trapnell, C. Defining cell types and states with single-cell genomics. Genome Res. 25, 1491–1498 (2015).
Kiselev, V. Y. et al. SC3: consensus clustering of single-cell RNA-seq data. Nat. Methods 14, 483–486 (2017).
Hao, Y. et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 42, 293–304 (2024).
Nayak, R. & Hasija, Y. A hitchhiker’s guide to single-cell transcriptomics and data analysis pipelines. Genomics 113, 606–619 (2021).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Dominguez Conde, C. et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science 376, eabl5197 (2022).
Xu, C. et al. Automatic cell-type harmonization and integration across Human Cell Atlas datasets. Cell 186, 5876–5891 (2023).
Menon, V. Clustering single cells: a review of approaches on high-and low-depth single-cell RNA-seq data. Brief. Funct. Genomics 17, 240–245 (2018).
Pancheva, A., Wheadon, H., Rogers, S. & Otto, T. D. Using topic modeling to detect cellular crosstalk in scRNA-seq. PLoS Comput. Biol. 18, e1009975 (2022).
Sokol, L. et al. Prioritization and functional validation of target genes from single-cell transcriptomics studies. Commun. Biol. 6, 648 (2023).
Lahnemann, D. et al. Eleven grand challenges in single-cell data science. Genome Biol. 21, 31 (2020).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 (2021).
Ding, J. et al. Systematic comparison of single-cell and single-nucleus RNA-sequencing methods. Nat. Biotechnol. 38, 737–746 (2020).
Zeng, H. & Sanes, J. R. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat. Rev. Neurosci. 18, 530–546 (2017).
Scala, F. et al. Phenotypic variation of transcriptomic cell types in mouse motor cortex. Nature 598, 144–150 (2021). This paper applies Patch-seq to explore the connection between single-cell gene expression, morphology and electrophysiological properties in neurons of the mouse motor cortex, illustrating the complex relationship between a cell’s transcriptional subtype and its functional properties.
Clark, I. C. et al. Identification of astrocyte regulators by nucleic acid cytometry. Nature 614, 326–333 (2023).
Jung, N. & Kim, T. K. Spatial transcriptomics in neuroscience. Exp. Mol. Med 55, 2105–2115 (2023).
Fangma, Y., Liu, M., Liao, J., Chen, Z. & Zheng, Y. Dissecting the brain with spatially resolved multi-omics. J. Pharm. Anal. 13, 694–710 (2023).
Hartman, A. & Satija, R. Comparative analysis of multiplexed in situ gene expression profiling technologies. eLife 13, RP96949 (2024).
Rodriques, S. G. et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019).
Russell, A. J. C. et al. Slide-tags enables single-nucleus barcoding for multimodal spatial genomics. Nature 625, 101–109 (2024). This paper describes a new approach for spatial single-nucleus genomic profiling in frozen tissue sections using spatial barcoding, which can be applied to multiple modalities including snRNA-seq, ATAC-seq and T cell antigen receptor sequencing. This tool can be applied for multimodal, orthogonal discovery and validation experiments.
Chen, K. H., Boettiger, A. N., Moffitt, J. R., Wang, S. & Zhuang, X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Wang, X. et al. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 361, eaat5691 (2018).
Ke, R. et al. In situ sequencing for RNA analysis in preserved tissue and cells. Nat. Methods 10, 857–860 (2013).
Liu, Y., Beyer, A. & Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 165, 535–550 (2016).
Wegler, C. et al. Global variability analysis of mRNA and protein concentrations across and within human tissues. NAR Genom. Bioinform 2, lqz010 (2020).
Buccitelli, C. & Selbach, M. mRNAs, proteins and the emerging principles of gene expression control. Nat. Rev. Genet. 21, 630–644 (2020).
Almet, A. A., Cang, Z., Jin, S. & Nie, Q. The landscape of cell-cell communication through single-cell transcriptomics. Curr. Opin. Syst. Biol. 26, 12–23 (2021).
Dimitrov, D. et al. Comparison of methods and resources for cell-cell communication inference from single-cell RNA-seq data. Nat. Commun. 13, 3224 (2022).
Armingol, E., Officer, A., Harismendy, O. & Lewis, N. E. Deciphering cell-cell interactions and communication from gene expression. Nat. Rev. Genet. 22, 71–88 (2021).
Gault, J. et al. Combining native and ‘omics’ mass spectrometry to identify endogenous ligands bound to membrane proteins. Nat. Methods 17, 505–508 (2020).
Wang, J. Y. & Doudna, J. A. CRISPR technology: a decade of genome editing is only the beginning. Science 379, eadd8643 (2023).
Gilbert, L. A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
Kearns, N. A. et al. Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nat. Methods 12, 401–403 (2015).
Thakore, P. I. et al. Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat. Methods 12, 1143–1149 (2015).
Adamson, B. et al. A multiplexed single-cell CRISPR screening platform enables systematic dissection of the unfolded protein response. Cell 167, 1867–1882 (2016).
Dixit, A. et al. Perturb-seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 167, 1853–1866 (2016).
Datlinger, P. et al. Pooled CRISPR screening with single-cell transcriptome readout. Nat. Methods 14, 297–301 (2017).
Wheeler, M. A. et al. Droplet-based forward genetic screening of astrocyte-microglia cross-talk. Science 379, 1023–1030 (2023).
Wu, D. et al. Dual genome-wide coding and lncRNA screens in neural induction of induced pluripotent stem cells. Cell Genom. 2, 100177 (2022).
Cooper, Y. A. et al. Functional regulatory variants implicate distinct transcriptional networks in dementia. Science 377, eabi8654 (2022).
Yang, X. et al. Functional characterization of Alzheimer’s disease genetic variants in microglia. Nat. Genet. 55, 1735–1744 (2023).
Tian, R. et al. CRISPR interference-based platform for multimodal genetic screens in human iPSC-derived neurons. Neuron 104, 239–255 (2019).
Drager, N. M. et al. A CRISPRi/a platform in human iPSC-derived microglia uncovers regulators of disease states. Nat. Neurosci. 25, 1149–1162 (2022). This paper uses a multiplex CRISPRi and CRISPRa platform using iPS cell microglia to identify genes that alter microglial cell states and functions when perturbed. It also uses scRNA-seq and dataset integration to demonstrate that microglial states in this in vitro system replicate states observed in postmortem human brain tissue.
Leng, K. et al. CRISPRi screens in human iPSC-derived astrocytes elucidate regulators of distinct inflammatory reactive states. Nat. Neurosci. 25, 1528–1542 (2022).
Li, E. et al. CRISPRi-based screens in iAssembloids to elucidate neuron-glia interactions. Preprint at bioRxiv https://doi.org/10.1101/2023.04.26.538498 (2023).
Esk, C. et al. A human tissue screen identifies a regulator of ER secretion as a brain-size determinant. Science 370, 935–941 (2020).
Jin, X. et al. In vivo Perturb-seq reveals neuronal and glial abnormalities associated with autism risk genes. Science 370, eaaz6063 (2020).
Wertz, M. H. et al. Genome-wide in vivo CNS screening identifies genes that modify CNS neuronal survival and mHTT toxicity. Neuron 106, 76–89 (2020).
Ramani, B. et al. Scalable, cell type-selective, AAV-based in vivo CRISPR screening in the mouse brain. Preprint at bioRxiv https://doi.org/10.1101/2023.06.13.544831 (2023).
Wheeler, M. A. et al. Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell 176, 581–596 (2019).
Hasel, P., Rose, I. V. L., Sadick, J. S., Kim, R. D. & Liddelow, S. A. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat. Neurosci. 24, 1475–1487 (2021).
Buettner, F. et al. Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Nat. Biotechnol. 33, 155–160 (2015).
Velten, L. et al. Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol. 19, 271–281 (2017).
Crow, M., Paul, A., Ballouz, S., Huang, Z. J. & Gillis, J. Characterizing the replicability of cell types defined by single cell RNA-sequencing data using MetaNeighbor. Nat. Commun. 9, 884 (2018).
Brunner, G., Lang, K., Wolfe, R. A., McClure, D. B. & Sato, G. H. Selective cell culture of brain cells by serum-free, hormone-supplemented media: a comparative morphological study. Brain Res. 254, 563–575 (1981).
Foo, L. C. et al. Development of a method for the purification and culture of rodent astrocytes. Neuron 71, 799–811 (2011).
Bohlen, C. J., Bennett, F. C. & Bennett, M. L. Isolation and culture of microglia. Curr. Protoc. Immunol. 125, e70 (2019).
Guttenplan, K. A. & Liddelow, S. A. Astrocytes and microglia: models and tools. J. Exp. Med. 216, 71–83 (2019).
Hall, C. E. et al. Progressive motor neuron pathology and the role of astrocytes in a human stem cell model of VCP-related ALS. Cell Rep. 19, 1739–1749 (2017).
de Majo, M. et al. Granulin loss of function in human mature brain organoids implicates astrocytes in TDP-43 pathology. Stem Cell Rep. 18, 706–719 (2023).
Cuomo, A. S. E. et al. Single-cell RNA-sequencing of differentiating iPS cells reveals dynamic genetic effects on gene expression. Nat. Commun. 11, 810 (2020).
Virdi, G. S. et al. Protein aggregation and calcium dysregulation are hallmarks of familial Parkinson’s disease in midbrain dopaminergic neurons. NPJ Parkinsons Dis. 8, 162 (2022).
Szebenyi, K. et al. Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology. Nat. Neurosci. 24, 1542–1554 (2021).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). One of the first studies to develop an in vitro functional testing platform to interrogate and validate a transcriptomically defined reactive astrocyte substate.
Barbar, L. et al. CD49f is a novel marker of functional and reactive human iPSC-derived astrocytes. Neuron 107, 436–453 (2020).
Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, abf1230 (2021).
Pasqual, G. et al. Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling. Nature 553, 496–500 (2018).
Gerfen, C. R., Paletzki, R. & Heintz, N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 80, 1368–1383 (2013).
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003).
Gertler, T. S., Chan, C. S. & Surmeier, D. J. Dichotomous anatomical properties of adult striatal medium spiny neurons. J. Neurosci. 28, 10814–10824 (2008).
Fenno, L. E. et al. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772 (2014).
Kramer, D. J. et al. Generation of a DAT-P2A-Flpo mouse line for intersectional genetic targeting of dopamine neuron subpopulations. Cell Rep. 35, 109123 (2021).
Poulin, J. F. et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 21, 1260–1271 (2018).
Ren, J. et al. Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. Elife 8, e49424 (2019).
Okaty, B. W. et al. A single-cell transcriptomic and anatomic atlas of mouse dorsal raphe Pet1 neurons. Elife 9, e55523 (2020).
BRAIN Initiative Cell Census Network. A multimodal cell census and atlas of the mammalian primary motor cortex. Nature 598, 86–102 (2021).
Cadwell, C. R. et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat. Biotechnol. 34, 199–203 (2016). A technical improvement is provided with the advent of PATCH-seq—enabling the electrophysiological recording and transcriptomic measurement in individual neurons.
Fuzik, J. et al. Integration of electrophysiological recordings with single-cell RNA-seq data identifies neuronal subtypes. Nat. Biotechnol. 34, 175–183 (2016).
Sloan, S. A. et al. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779–790 (2017).
Xu, S. et al. Behavioral state coding by molecularly defined paraventricular hypothalamic cell type ensembles. Science 370, eabb2494 (2020).
Condylis, C. et al. Dense functional and molecular readout of a circuit hub in sensory cortex. Science 375, eabl5981 (2022).
Bugeon, S. et al. A transcriptomic axis predicts state modulation of cortical interneurons. Nature 607, 330–338 (2022).
Guttenplan, K. A. et al. Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nat. Commun. 11, 3753 (2020).
Guttenplan, K. A. et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature https://doi.org/10.1038/s41586-021-03960-y (2021).
Zeng, H. What is a cell type and how to define it? Cell 185, 2739–2755 (2022).
Yuste, R. et al. A community-based transcriptomics classification and nomenclature of neocortical cell types. Nat. Neurosci. 23, 1456–1468 (2020).
Hodge, R. D. et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68 (2019).
Arendt, D., Bertucci, P. Y., Achim, K. & Musser, J. M. Evolution of neuronal types and families. Curr. Opin. Neurobiol. 56, 144–152 (2019).
Wagner, D. E. & Klein, A. M. Lineage tracing meets single-cell omics: opportunities and challenges. Nat. Rev. Genet. 21, 410–427 (2020).
Bandler, R. C. et al. Single-cell delineation of lineage and genetic identity in the mouse brain. Nature 601, 404–409 (2022).
Cadwell, C. R. et al. Cell type composition and circuit organization of clonally related excitatory neurons in the juvenile mouse neocortex. Elife 9, e52951 (2020).
Mayer, C. et al. Developmental diversification of cortical inhibitory interneurons. Nature 555, 457–462 (2018).
Nowakowski, T. J. et al. Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 358, 1318–1323 (2017).
Schmitz, M. T. et al. The development and evolution of inhibitory neurons in primate cerebrum. Nature 603, 871–877 (2022). This paper uses comparative single-cell genomics in mammalian brains over developmental stages, identifying the evolutionary changes in developmental specification of a class of primate-specific interneurons.
Woych, J. et al. Cell-type profiling in salamanders identifies innovations in vertebrate forebrain evolution. Science 377, eabp9186 (2022).
Hahn, J. et al. Evolution of neuronal cell classes and types in the vertebrate retina. Nature 624, 415–424 (2023).
Krienen, F. M. et al. Innovations present in the primate interneuron repertoire. Nature 586, 262–269 (2020).
Tosches, M. A. et al. Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360, 881–888 (2018).
Peng, Y. R. et al. Molecular classification and comparative taxonomics of foveal and peripheral cells in primate retina. Cell 176, 1222–1237 (2019).
Jorstad, N. L. et al. Comparative transcriptomics reveals human-specific cortical features. Science 382, eade9516 (2023).
Hain, D. et al. Molecular diversity and evolution of neuron types in the amniote brain. Science 377, eabp8202 (2022). This paper shows that generating single-cell genomics brain atlases from non-model organisms (that is, an exemplar lizard) and comparing to mammals can identify conserved neuronal cell types throughout the brains of both species across hundreds of million years of evolution while also showing how evolution has modified this conservation based on connectivity needs.
Lust, K. et al. Single-cell analyses of axolotl telencephalon organization, neurogenesis, and regeneration. Science 377, eabp9262 (2022).
Tarashansky, A. J. et al. Mapping single-cell atlases throughout metazoa unravels cell type evolution. Elife 10, e66747 (2021).
Chen, J. et al. A quantitative framework for characterizing the evolutionary history of mammalian gene expression. Genome Res. 29, 53–63 (2019).
Bakken, T. E. et al. Comparative cellular analysis of motor cortex in human, marmoset and mouse. Nature 598, 111–119 (2021). This paper used multimodal single-cell approaches (snRNA-seq, snATAC-seq, single-cell methylation, morphology and Patch-seq) across mammalian species to determine the species-specific adaptiations of cell types in one brain region and focuses on differences in Betz cells.
Caglayan, E. et al. Molecular features driving cellular complexity of human brain evolution. Nature 620, 145–153 (2023). This paper demonstrates the power of comparing proportions of nonneuronal cell types (that is, the oligodendrocyte lineage) across closely related primate species and the integration of ancient human genomes to identify modern human-specific variants associated with human-specific chromatin accessibility.
Jeon, H. et al. Statistical power analysis for designing bulk, single-cell, and spatial transcriptomics experiments: review, tutorial, and perspectives. Biomolecules 13, 221 (2023).
Svensson, V. et al. Power analysis of single-cell RNA-sequencing experiments. Nat. Methods 14, 381–387 (2017).
Su, K., Wu, Z. & Wu, H. Simulation, power evaluation and sample size recommendation for single-cell RNA-seq. Bioinformatics 36, 4860–4868 (2020).
Arendt, D. et al. The origin and evolution of cell types. Nat. Rev. Genet. 17, 744–757 (2016).
Colquitt, B. M., Merullo, D. P., Konopka, G., Roberts, T. F. & Brainard, M. S. Cellular transcriptomics reveals evolutionary identities of songbird vocal circuits. Science 371, eabd9704 (2021).
Kebschull, J. M. et al. Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science 370, abd5059 (2020).
Lamanna, F. et al. A lamprey neural cell type atlas illuminates the origins of the vertebrate brain. Nat. Ecol. Evol. 7, 1714–1728 (2023).
Shafer, M. E. R., Sawh, A. N. & Schier, A. F. Gene family evolution underlies cell-type diversification in the hypothalamus of teleosts. Nat. Ecol. Evol. 6, 63–76 (2022).
Tarashansky, A. J., Xue, Y., Li, P., Quake, S. R. & Wang, B. Self-assembling manifolds in single-cell RNA sequencing data. Elife 8, e48994 (2019).
Agboola, O. S., Hu, X., Shan, Z., Wu, Y. & Lei, L. Brain organoid: a 3D technology for investigating cellular composition and interactions in human neurological development and disease models in vitro. Stem Cell Res. Ther. 12, 430 (2021).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Amiri, A. et al. Transcriptome and epigenome landscape of human cortical development modeled in organoids. Science 362, eaat6720 (2018).
Bhaduri, A. et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature 578, 142–148 (2020).
Camp, J. G. et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl Acad. Sci. USA 112, 15672–15677 (2015).
Gordon, A. et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 24, 331–342 (2021).
Pollen, A. A. et al. Establishing cerebral organoids as models of human-specific brain evolution. Cell 176, 743–756 (2019).
Velasco, S. et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570, 523–527 (2019).
Qian, X. et al. Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell 26, 766–781 (2020).
Xiang, Y. et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21, 383–398 (2017).
Pinson, A. et al. Human TKTL1 implies greater neurogenesis in frontal neocortex of modern humans than Neanderthals. Science 377, eabl6422 (2022).
Trujillo, C. A. et al. Reintroduction of the archaic variant of NOVA1 in cortical organoids alters neurodevelopment. Science 371, eaax2537 (2021).
Kanton, S. et al. Organoid single-cell genomic atlas uncovers human-specific features of brain development. Nature 574, 418–422 (2019).
Uzquiano, A. et al. Proper acquisition of cell class identity in organoids allows definition of fate specification programs of the human cerebral cortex. Cell 185, 3770–3788 (2022).
Fleck, J. S. et al. Inferring and perturbing cell fate regulomes in human brain organoids. Nature https://doi.org/10.1038/s41586-022-05279-8 (2022).
Bershteyn, M. et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 20, 435–449 (2017).
Trevino, A. E. et al. Chromatin accessibility dynamics in a model of human forebrain development. Science 367, eaay1645 (2020).
Li, C. et al. Single-cell brain organoid screening identifies developmental defects in autism. Nature 621, 373–380 (2023).
Mora-Bermudez, F. et al. Differences and similarities between human and chimpanzee neural progenitors during cerebral cortex development. Elife 5, e18683 (2016).
Tanaka, Y., Cakir, B., Xiang, Y., Sullivan, G. J. & Park, I. H. Synthetic analyses of single-cell transcriptomes from multiple brain organoids and fetal brain. Cell Rep. 30, 1682–1689 (2020).
Werner, J. M. & Gillis, J. Preservation of co-expression defines the primary tissue fidelity of human neural organoids. Preprint at bioRxiv https://doi.org/10.1101/2023.03.31.535112 (2023).
Czerminski, J. T., King, O. D. & Lawrence, J. B. Large-scale organoid study suggests effects of trisomy 21 on early fetal neurodevelopment are more subtle than variability between isogenic lines and experiments. Front. Neurosci. 16, 972201 (2022).
Lee, J. H. et al. Cell-line dependency in cerebral organoid induction: cautionary observations in Alzheimer’s disease patient-derived induced pluripotent stem cells. Mol. Brain 15, 46 (2022).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Haghverdi, L., Lun, A. T. L., Morgan, M. D. & Marioni, J. C. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol. 36, 421–427 (2018).
Johansen, N. & Quon, G. scAlign: a tool for alignment, integration, and rare cell identification from scRNA-seq data. Genome Biol. 20, 166 (2019).
Polanski, K. et al. BBKNN: fast batch alignment of single cell transcriptomes. Bioinformatics 36, 964–965 (2020).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Welch, J. D. et al. Single-cell multi-omic integration compares and contrasts features of brain cell identity. Cell 177, 1873–1887 (2019).
Mostajo-Radji, M. A., Schmitz, M. T., Montoya, S. T. & Pollen, A. A. Reverse engineering human brain evolution using organoid models. Brain Res. 1729, 146582 (2020).
Krienen, F. M. et al. A marmoset brain cell census reveals regional specialization of cellular identities. Sci Adv. 9, eadk3986 (2023).
Caglayan, E., Liu, Y. & Konopka, G. Neuronal ambient RNA contamination causes misinterpreted and masked cell types in brain single-nuclei datasets. Neuron 110, 4043–4056 (2022).
Soreq, L. et al. Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep. 18, 557–570 (2017).
Norimoto, H. et al. A claustrum in reptiles and its role in slow-wave sleep. Nature 578, 413–418 (2020).
Marsh, S. E. et al. Dissection of artifactual and confounding glial signatures by single-cell sequencing of mouse and human brain. Nat. Neurosci. 25, 306–316 (2022).
Acknowledgements
We thank I. Adameyko, T. Bakken, A. Bhaduri, B. Bonev, G. Castelo-Branco, F. Chen, C. Chhatbar, S. Codeluppi, R. Corces, J. Fan, M. G. Filbin, D. Gate, G. Green, M. Heiman, K. Harris, H. Hochgerner, F. Inoue, M. Kellis, C. N. Kim, J. Krull, G. L. Manno, A. Levine, Q. Li, S. Linnarsson, M. Lotfollahi, C. Luo, Q. Ma, E. Macosko, C. Mayer, K. R. Maynard, V. Menon, P. Nano, M. Nitzan, M. Prinz, S. Quake, V. Ramani, R. Satijia, L. Schirmer, Y. Shen, N. Sun, F. Theis, C. Walsh, X. Wang, J. D. Welch and J. Yang for the insightful feedback on this work. M.C. thanks A. U. Antonova for helpful additional discussions. G.K. is a Jon Heighten Scholar in Autism Research and Townsend Distinguished Chair in Research on Autism Spectrum Disorders at UT Southwestern, and was partially supported by the James S. McDonnell Foundation 21st Century Science Initiative in Understanding Human Cognition Scholar Award (220020467), Simons Foundation for Autism Research Award (947591) and National Institutes of Health (NIH) grants (NS126143, HG011641, MH126481, NS115821). S.A.L. is supported by the Carol and Gene Ludwig Family Foundation, The Cure Alzheimer’s Fund, the National Multiple Sclerosis Society and NIH grants (R01EY033353, R03NS127079). C.R.C. is supported by the Weill Neurohub, the Shurl and Kay Curci Foundation, the CURE Epilepsy Taking Flight Award and grants from the NIH (K08NS126573, U01NS132353). E.C. is a Neural Scientist Training Program Fellow in the Peter O’Donnell Brain Institute at UT Southwestern. J.L.C. is supported by NIH grant U01MH10907. J.G. is supported by NIH grant R01MH113005. M.S. is supported by a HHMI Hanna H. Gray Fellowship, a SFARI Pilot Award and a NYSCF Druckenmiller Fellowship. J.W. is supported by NIH grant R01MH113005. M.E.U. is supported by the National Science Foundation Graduate Research Fellowship Program and a Hertz Foundation Fellowship Program.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of this Review.
Corresponding authors
Ethics declarations
Competing interests
M.C. is a member of the Scientific Advisory Board of Vigil, NGMBio, Cartesian and Halyardtx. M.C. receives research support from Ono Pharmaceutical, is a consultant for CST and has patents pending on LILRB4 and TREM2. S.A.L. maintains a financial interest in AstronauTx and Synapticure and is on the Scientific Advisory Board of the Global BioAccess Fund. S.A.L. is an inventor on US Patents WO2018081250A1 and WO2022187517A1. M.K. is a co-scientific founder of Montara Therapeutics and serves on the Scientific Advisory Boards of Engine Biosciences, Casma Therapeutics, Cajal Neuroscience, Alector and Montara Therapeutics, and is an advisor to Modulo Bio and Recursion Therapeutics. M.K. is an inventor on US Patent 11,254,933 related to CRISPRi and CRISPRa screening, and on a US Patent Application on in vivo screening methods. All other authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Renzo Mancuso and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Colonna, M., Konopka, G., Liddelow, S.A. et al. Implementation and validation of single-cell genomics experiments in neuroscience. Nat Neurosci 27, 2310–2325 (2024). https://doi.org/10.1038/s41593-024-01814-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41593-024-01814-0
This article is cited by
-
Aging in mice alters regionally enriched striatal astrocytes
Nature Communications (2025)
-
The new frontier in understanding human and mammalian brain development
Nature (2025)
-
Focus on single-cell genomics
Nature Neuroscience (2024)
-
Opportunities and challenges of single-cell and spatially resolved genomics methods for neuroscience discovery
Nature Neuroscience (2024)
-
Applying single-cell and single-nucleus genomics to studies of cellular heterogeneity and cell fate transitions in the nervous system
Nature Neuroscience (2024)