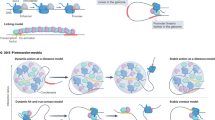
Abstract
How enhancers communicate with their target genes to influence transcription is an unresolved question of fundamental importance. Current models of the mechanism of enhancer–target gene or enhancer–promoter (E–P) communication are transcription-factor-centric and underappreciate major findings, including that enhancers are themselves transcribed by RNA polymerase II, which correlates with enhancer activity. In this Perspective, we posit that enhancer transcription and its products, enhancer RNAs, are elementary components of enhancer–gene communication. Specifically, we discuss the possibility that transcription at enhancers and at their cognate genes are linked and that this coupling is at the basis of how enhancers communicate with their targets. This model of transcriptional coupling between enhancers and their target genes is supported by growing experimental evidence and represents a synthesis of recent key discoveries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bradner, J. E., Hnisz, D. & Young, R. A. Transcriptional addiction in cancer. Cell 168, 629–643 (2017).
Wissink, E. M., Vihervaara, A., Tippens, N. D. & Lis, J. T. Nascent RNA analyses: tracking transcription and its regulation. Nat. Rev. Genet. 20, 705–723 (2019).
Bhuiyan, T. & Timmers, H. T. M. Promoter recognition: putting TFIID on the spot. Trends Cell Biol. 29, 752–763 (2019).
Vos, S. M. et al. Structure of activated transcription complex Pol II–DSIF–PAF–SPT6. Nature 560, 607–612 (2018).
Kireeva, M. L., Komissarova, N., Waugh, D. S. & Kashlev, M. The 8-nucleotide-long RNA:DNA hybrid is a primary stability determinant of the RNA polymerase II elongation complex. J. Biol. Chem. 275, 6530–6536 (2000).
Fong, N. et al. Effects of transcription elongation rate and Xrn2 exonuclease activity on RNA polymerase II termination suggest widespread kinetic competition. Mol. Cell 60, 256–267 (2015).
Schwalb, B. et al. TT-seq maps the human transient transcriptome. Science 352, 1225–1228 (2016).
Chen, F. X., Smith, E. R. & Shilatifard, A. Born to run: control of transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 19, 464–478 (2018).
Core, L. & Adelman, K. Promoter-proximal pausing of RNA polymerase II: a nexus of gene regulation. Genes Dev. 33, 960–982 (2019).
Gonzalez, M. N., Blears, D. & Svejstrup, J. Q. Causes and consequences of RNA polymerase II stalling during transcript elongation. Nat. Rev. Mol. Cell Biol. 22, 3–21 (2021).
Thomas, H. F. & Buecker, C. What is an enhancer? BioEssays 45, e2300044 (2023).
Furlong, E. E. M. & Levine, M. Developmental enhancers and chromosome topology. Science 361, 1341–1345 (2018).
Schoenfelder, S. & Fraser, P. Long-range enhancer–promoter contacts in gene expression control. Nat. Rev. Genet. 20, 437–455 (2019).
Beagrie, R. A. & Pombo, A. Gene activation by metazoan enhancers: diverse mechanisms stimulate distinct steps of transcription. BioEssays 38, 881–893 (2016).
Szutorisz, H., Dillon, N. & Tora, L. The role of enhancers as centres for general transcription factor recruitment. Trends Biochem. Sci. 30, 593–599 (2005).
Liu, W. et al. Brd4 and JMJD6-associated anti-pause enhancers in regulation of transcriptional pause release. Cell 155, 1581–1595 (2013).
Ghavi-Helm, Y. et al. Enhancer loops appear stable during development and are associated with paused polymerase. Nature 512, 96–100 (2014).
Chen, F. X. et al. PAF1 regulation of promoter-proximal pause release via enhancer activation. Science 357, 1294–1298 (2017).
Meng, H. & Bartholomew, B. Emerging roles of transcriptional enhancers in chromatin looping and promoter-proximal pausing of RNA polymerase II. J. Biol. Chem. 293, 13786–13794 (2018).
McCord, R. P., Kaplan, N. & Giorgetti, L. Chromosome conformation capture and beyond: toward an integrative view of chromosome structure and function. Mol. Cell 77, 688–708 (2020).
Hafner, A. & Boettiger, A. The spatial organization of transcriptional control. Nat. Rev. Genet. 24, 53–68 (2023).
Robson, M. I., Ringel, A. R. & Mundlos, S. Regulatory landscaping: how enhancer–promoter communication is sculpted in 3D. Mol. Cell 74, 1110–1122 (2019).
Panne, D. The enhanceosome. Curr. Opin. Struct. Biol. 18, 236–242 (2008).
Karr, J. P., Ferrie, J. J., Tjian, R. & Darzacq, X. The transcription factor activity gradient (TAG) model: contemplating a contact-independent mechanism for enhancer–promoter communication. Genes Dev. 36, 7–16 (2021).
Zabidi, M. A. & Stark, A. Regulatory enhancer–core–promoter communication via transcription factors and cofactors. Trends Genet. 32, 801–814 (2016).
Kim, S. & Wysocka, J. Deciphering the multi-scale, quantitative cis-regulatory code. Mol. Cell 83, 373–392 (2023).
Li, J. & Pertsinidis, A. New insights into promoter–enhancer communication mechanisms revealed by dynamic single-molecule imaging. Biochem. Soc. Trans. 49, 1299–1309 (2021).
Yang, J. H. & Hansen, A. S. Enhancer selectivity in space and time: from enhancer–promoter interactions to promoter activation. Nat. Rev. Mol. Cell Biol. 25, 574–591 (2024).
Popay, T. M. & Dixon, J. R. Coming full circle: on the origin and evolution of the looping model for enhancer–promoter communication. J. Biol. Chem. 298, 102117 (2022).
Zuin, J. et al. Nonlinear control of transcription through enhancer–promoter interactions. Nature 604, 571–577 (2022).
Hnisz, D., Shrinivas, K., Young, R. A., Chakraborty, A. K. & Sharp, P. A. A phase separation model for transcriptional control. Cell 169, 13–23 (2017).
Lim, B. & Levine, M. S. Enhancer–promoter communication: hubs or loops? Curr. Opin. Genet. Dev. 67, 5–9 (2021).
Vernimmen, D. & Bickmore, W. A. The hierarchy of transcriptional activation: from enhancer to promoter. Trends Genet. 31, 696–708 (2015).
Lee, K., Hsiung, C. C.-S., Huang, P., Raj, A. & Blobel, G. A. Dynamic enhancer–gene body contacts during transcription elongation. Genes Dev. 29, 1992–1997 (2015).
Beagrie, R. A. et al. Complex multi-enhancer contacts captured by genome architecture mapping. Nature 543, 519–524 (2017).
Bressin, A. et al. High-sensitive nascent transcript sequencing reveals BRD4-specific control of widespread enhancer and target gene transcription. Nat. Commun. 14, 4971 (2023).
Santa, F. D. et al. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 8, e1000384 (2010).
Kim, T.-K. et al. Widespread transcription at neuronal activity-regulated enhancers. Nature 465, 182–187 (2010).
Koch, F. et al. Transcription initiation platforms and GTF recruitment at tissue-specific enhancers and promoters. Nat. Struct. Mol. Biol. 18, 956–963 (2011).
Core, L. J. et al. Analysis of nascent RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. Nat. Genet. 46, 1311–1320 (2014).
Andersson, R. et al. An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461 (2014).
Henriques, T. et al. Widespread transcriptional pausing and elongation control at enhancers. Genes Dev. 32, 26–41 (2018).
Mikhaylichenko, O. et al. The degree of enhancer or promoter activity is reflected by the levels and directionality of eRNA transcription. Genes Dev. 32, 42–57 (2018).
Hah, N., Murakami, S., Nagari, A., Danko, C. G. & Kraus, W. L. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 23, 1210–1223 (2013).
Tippens, N. D. et al. Transcription imparts architecture, function and logic to enhancer units. Nat. Genet. 52, 1067–1075 (2020).
Raisner, R. et al. Enhancer activity requires CBP/P300 bromodomain-dependent histone H3K27 acetylation. Cell Rep. 24, 1722–1729 (2018).
Rada-Iglesias, A. et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283 (2011).
Creyghton, M. P. et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl Acad. Sci. USA 107, 21931–21936 (2010).
Kim, T.-K. & Shiekhattar, R. Architectural and functional commonalities between enhancers and promoters. Cell 162, 948–959 (2015).
Andersson, R., Sandelin, A. & Danko, C. G. A unified architecture of transcriptional regulatory elements. Trends Genet. 31, 426–433 (2015).
Lai, F., Gardini, A., Zhang, A. & Shiekhattar, R. Integrator mediates the biogenesis of enhancer RNAs. Nature 525, 399–403 (2015).
Wagner, E. J., Tong, L. & Adelman, K. Integrator is a global promoter-proximal termination complex. Mol. Cell 83, 416–427 (2023).
Narita, T. et al. Enhancers are activated by p300/CBP activity-dependent PIC assembly, RNAPII recruitment, and pause release. Mol. Cell 81, 2166–2182 (2021).
Dukler, N. et al. Nascent RNA sequencing reveals a dynamic global transcriptional response at genes and enhancers to the natural medicinal compound celastrol. Genome Res. 27, 1816–1829 (2017).
Tippens, N. D., Vihervaara, A. & Lis, J. T. Enhancer transcription: what, where, when, and why? Genes Dev. 32, 1–3 (2018).
Dao, L. T. M. et al. Genome-wide characterization of mammalian promoters with distal enhancer functions. Nat. Genet. 49, 1073–1081 (2017).
Kowalczyk, M. S. et al. Intragenic enhancers act as alternative promoters. Mol. Cell 45, 447–458 (2012).
Li, G. et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98 (2012).
Natoli, G. & Andrau, J.-C. Noncoding transcription at enhancers: general principles and functional models. Annu. Rev. Genet. 46, 1–19 (2012).
Zhang, S. et al. PAX3–FOXO1 coordinates enhancer architecture, eRNA transcription, and RNA polymerase pause release at select gene targets. Mol. Cell 82, 4428–4442 (2022).
Hou, T. Y. & Kraus, W. L. Spirits in the material world: enhancer RNAs in transcriptional regulation. Trends Biochem. Sci 46, 138–153 (2021).
Kopp, F. & Mendell, J. T. Functional classification and experimental dissection of long noncoding RNAs. Cell 172, 393–407 (2018).
Gil, N. & Ulitsky, I. Production of spliced long noncoding RNAs specifies regions with increased enhancer activity. Cell Syst. 7, 537–547 (2018).
Chen, Q. et al. Enhancer RNAs in transcriptional regulation: recent insights. Front. Cell Dev. Biol. 11, 1205540 (2023).
Sartorelli, V. & Lauberth, S. M. Enhancer RNAs are an important regulatory layer of the epigenome. Nat. Struct. Mol. Biol. 27, 521–528 (2020).
Li, W. et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 498, 516–520 (2013).
Xiang, J.-F. et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 24, 513–531 (2014).
Hsieh, C.-L. et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc. Natl Acad. Sci. USA 111, 7319–7324 (2014).
Tan, S. H. et al. The enhancer RNA ARIEL activates the oncogenic transcriptional program in T-cell acute lymphoblastic leukemia. Blood 134, 239–251 (2019).
Sigova, A. A. et al. Transcription factor trapping by RNA in gene regulatory elements. Science 350, 978–981 (2015).
Rahnamoun, H. et al. RNAs interact with BRD4 to promote enhanced chromatin engagement and transcription activation. Nat. Struct. Mol. Biol. 25, 687–697 (2018).
Mousavi, K. et al. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol. Cell 51, 606–617 (2013).
Tsai, P.-F. et al. A muscle-specific enhancer RNA mediates cohesin recruitment and regulates transcription in trans. Mol. Cell 71, 129–141 (2018).
Bose, D. A. et al. RNA binding to CBP stimulates histone acetylation and transcription. Cell 168, 135–149 (2017).
Saha, D. et al. Enhancer switching in cell lineage priming is linked to eRNA, Brg1’s AT-hook, and SWI/SNF recruitment. Mol. Cell 84, 1855–1869 (2024).
Shi, L. et al. Enhancer RNA and NFκB-dependent P300 regulation of ADAMDEC1. Mol. Immunol. 103, 312–321 (2018).
Hou, T. Y. & Kraus, W. L. Analysis of estrogen-regulated enhancer RNAs identifies a functional motif required for enhancer assembly and gene expression. Cell Rep. 39, 110944 (2022).
Zhao, Y. et al. Activation of P-TEFb by Androgen Receptor-Regulated Enhancer RNAs in Castration-Resistant Prostate Cancer. Cell Rep. 15, 599–610 (2016).
Zhao, Y. et al. MyoD induced enhancer RNA interacts with hnRNPL to activate target gene transcription during myogenic differentiation. Nat. Commun. 10, 5787 (2019).
Schaukowitch, K. et al. Enhancer RNA Facilitates NELF Release from immediate early genes. Mol. Cell 56, 29–42 (2014).
Gorbovytska, V. et al. Enhancer RNAs stimulate Pol II pause release by harnessing multivalent interactions to NELF. Nat. Commun. 13, 2429 (2022).
Altendorfer, E., Mochalova, Y. & Mayer, A. BRD4: a general regulator of transcription elongation. Transcription 13, 70–81 (2022).
Lee, J.-H. et al. Enhancer RNA m6A methylation facilitates transcriptional condensate formation and gene activation. Mol. Cell 81, 3368–3385 (2021).
Kaikkonen, M. U. et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol. Cell 51, 310–325 (2013).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Alexander, J. M. et al. Live-cell imaging reveals enhancer-dependent Sox2 transcription in the absence of enhancer proximity. eLife 8, e41769 (2019).
Benabdallah, N. S. et al. Decreased enhancer–promoter proximity accompanying enhancer activation. Mol. Cell 76, 473–484.e7 (2019).
Bialek, W., Gregor, T. & Tkačik, G. Action at a distance in transcriptional regulation. Preprint at https://doi.org/10.48550/arxiv.1912.08579 (2019).
Heist, T., Fukaya, T. & Levine, M. Large distances separate coregulated genes in living Drosophila embryos. Proc. Natl Acad. Sci. USA 116, 15062–15067 (2019).
Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 361, eaar3958 (2018).
Shrinivas, K. et al. Enhancer features that drive formation of transcriptional condensates. Mol. Cell 75, 549–561 (2019).
Cho, W.-K. et al. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 361, 412–415 (2018).
Williamson, I., Lettice, L. A., Hill, R. E. & Bickmore, W. A. Shh and ZRS enhancer colocalisation is specific to the zone of polarising activity. Development 143, 2994–3001 (2016).
Partridge, E. C. et al. Occupancy maps of 208 chromatin-associated proteins in one human cell type. Nature 583, 720–728 (2020).
Arnold, M. & Stengel, K. R. Emerging insights into enhancer biology and function. Transcription 14, 68–87 (2023).
Winter, G. E. et al. BET bromodomain proteins function as master transcription elongation factors independent of CDK9 recruitment. Mol. Cell 67, 5–18 (2017).
Zhang, W. et al. Bromodomain-containing protein 4 (BRD4) regulates RNA polymerase II serine 2 phosphorylation in human CD4+ T cells. J. Biol. Chem. 287, 43137–43155 (2012).
Kagey, M. H. et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature 467, 430–435 (2010).
Ramasamy, S. et al. The Mediator complex regulates enhancer-promoter interactions. Nat. Struct. Mol. Biol. 30, 991–1000 (2023).
Heintzman, N. D. et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39, 311–318 (2007).
Aguilo, F. et al. Deposition of 5-methylcytosine on enhancer RNAs enables the coactivator function of PGC-1α. Cell Rep. 14, 479–492 (2016).
Fitz, J. et al. Spt5-mediated enhancer transcription directly couples enhancer activation with physical promoter interaction. Nat. Genet. 52, 505–515 (2020).
Hu, S. et al. SPT5 stabilizes RNA polymerase II, orchestrates transcription cycles, and maintains the enhancer landscape. Mol. Cell 81, 4425–4439 (2021).
Gajos, M. et al. Conserved DNA sequence features underlie pervasive RNA polymerase pausing. Nucleic Acids Res. 49, 4402–4420 (2021).
Michel, M. et al. TT‐seq captures enhancer landscapes immediately after T‐cell stimulation. Mol. Syst. Biol. 13, 920 (2017).
Arner, E. et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 347, 1010–1014 (2015).
Hirabayashi, S. et al. NET-CAGE characterizes the dynamics and topology of human transcribed cis-regulatory elements. Nat. Genet. 51, 1369–1379 (2019).
Cheng, J.-H., Pan, D. Z.-C., Tsai, Z. T.-Y. & Tsai, H.-K. Genome-wide analysis of enhancer RNA in gene regulation across 12 mouse tissues. Sci. Rep. 5, 12648 (2015).
Chen, R. A.-J. et al. The landscape of RNA polymerase II transcription initiation in C. elegans reveals promoter and enhancer architectures. Genome Res. 23, 1339–1347 (2013).
Khor, J. M., Guerrero-Santoro, J., Douglas, W. & Ettensohn, C. A. Global patterns of enhancer activity during sea urchin embryogenesis assessed by eRNA profiling. Genome Res. 31, 1680–1692 (2021).
Kim, Y. J., Xie, P., Cao, L., Zhang, M. Q. & Kim, T. H. Global transcriptional activity dynamics reveal functional enhancer RNAs. Genome Res. 28, 1799–1811 (2018).
Hamamoto, K., Umemura, Y., Makino, S. & Fukaya, T. Dynamic interplay between non-coding enhancer transcription and gene activity in development. Nat. Commun. 14, 826 (2023).
Panigrahi, A. K. et al. SRC-3 coactivator governs dynamic estrogen-induced chromatin looping interactions during transcription. Mol. Cell 70, 679–694 (2018).
Panigrahi, A. K., Lonard, D. M. & O’Malley, B. W. Enhancer–promoter entanglement explains their transcriptional interdependence. Proc. Natl Acad. Sci. USA 120, e2216436120 (2023).
Melo, C. A. et al. eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol. Cell 49, 524–535 (2013).
Simeonov, D. R. et al. Discovery of stimulation-responsive immune enhancers with CRISPR activation. Nature 549, 111–115 (2017).
Li, K. et al. Interrogation of enhancer function by enhancer-targeting CRISPR epigenetic editing. Nat. Commun. 11, 485 (2020).
Carullo, N. V. N. et al. Enhancer RNAs predict enhancer–gene regulatory links and are critical for enhancer function in neuronal systems. Nucleic Acids Res. 48, gkaa671 (2020).
Nuñez, J. K. et al. Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing. Cell 184, 2503–2519 (2021).
Fulco, C. P. et al. Activity-by-contact model of enhancer–promoter regulation from thousands of CRISPR perturbations. Nat. Genet. 51, 1664–1669 (2019).
Xie, S., Duan, J., Li, B., Zhou, P. & Hon, G. C. Multiplexed engineering and analysis of combinatorial enhancer activity in single cells. Mol. Cell 66, 285–299 (2017).
Gasperini, M. et al. A genome-wide framework for mapping gene regulation via cellular genetic screens. Cell 176, 377–390 (2019).
Henninger, J. E. et al. RNA-mediated feedback control of transcriptional condensates. Cell 184, 207–225 (2021).
Lai, F. et al. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature 494, 497–501 (2013).
Sawada, T. et al. Androgen-dependent and DNA-binding-independent association of androgen receptor with chromatic regions coding androgen-induced noncoding RNAs. Biosci. Biotechnol. Biochem. 85, 2121–2130 (2021).
Nair, S. J. et al. Phase separation of ligand-activated enhancers licenses cooperative chromosomal enhancer assembly. Nat. Struct. Mol. Biol. 26, 193–203 (2019).
Ortega, E. et al. Transcription factor dimerization activates the p300 acetyltransferase. Nature 562, 538–544 (2018).
Nepal, C. et al. Ancestrally duplicated conserved noncoding element suggests dual regulatory roles of HOTAIR in cis and trans. iScience 23, 101008 (2020).
Lyons, H. et al. Functional partitioning of transcriptional regulators by patterned charge blocks. Cell 186, 327–345 (2023).
Fukaya, T., Lim, B. & Levine, M. Enhancer control of transcriptional bursting. Cell 166, 358–368 (2016).
Lim, B., Heist, T., Levine, M. & Fukaya, T. Visualization of transvection in living Drosophila embryos. Mol. Cell 70, 287–296 (2018).
Allou, L. et al. Non-coding deletions identify Maenli lncRNA as a limb-specific En1 regulator. Nature 592, 93–98 (2021).
Acknowledgements
We apologize to our colleagues whose work was not cited owing to space constraints. This Perspective was strongly improved by critical feedback from D. M. Ibrahim, L. Behrens, A. Bressin and G. J. Villafano. The original figures were created with Biorender.com. This work was supported by the Max Planck Society.
Author information
Authors and Affiliations
Contributions
E.A. prepared the original figures and helped write the manuscript. S.M. provided conceptual help. A.M. conceptualized the transcription coupling model and had overall responsibility for this work. A.M. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Eytan Zlotorynski & Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Box 1,
Supplementary Figures 1 and 2 and Supplementary References
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Altendorfer, E., Mundlos, S. & Mayer, A. A transcription coupling model for how enhancers communicate with their target genes. Nat Struct Mol Biol 32, 598–606 (2025). https://doi.org/10.1038/s41594-025-01523-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-025-01523-7
This article is cited by
-
Deciphering the regulatory landscape of enhancer RNAs in health and disease
Signal Transduction and Targeted Therapy (2026)
-
Engineering bidirectional chloroplast promoters for tunable co-expression of multiple genes in microalgae (Chlamydomonas reinhardtii)
Communications Biology (2026)
-
Closing 2025, and a look ahead
Nature Structural & Molecular Biology (2025)
-
Aberrant Enhancer Regulation, Phase Separation, and Autoimmune Diseases
Clinical Reviews in Allergy & Immunology (2025)