Figure 1
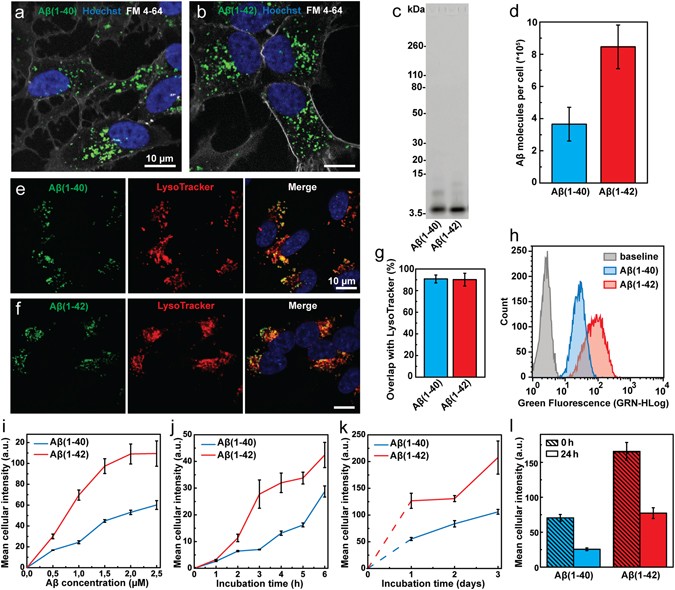
Imaging and quantification of the cellular uptake of Aβ(1–40) and Aβ(1–42) in SH-SY5Y cells. (a,b) Confocal microscopy images showing uptake of HF488-labelled Aβ(1–40) (a) and Aβ(1–42) (b) (green) after 24 h incubation at 500 nM peptide concentration. The cell nuclei (blue, Hoechst 33342) and outer cell membrane (grey, FM 4–64) were stained immediately prior to imaging. (c) SDS-PAGE of Aβ(1–40) and Aβ(1–42) solutions. The major band corresponds to HF488-labelled Aβ(1–40) and Aβ(1–42) monomers (4.7 and 4.9 kDa respectively). (d) Confocal imaging based quantification of the cellular uptake of Aβ(1–40) and Aβ(1–42) following incubation with 1 µM Aβ(1–40) or Aβ(1–42) for 8 h. The average uptake of Aβ molecules per cell was 3.7e5 ± 1.1e5 for Aβ(1–40) and 8.5e5 ± 1.6e5 for Aβ(1–42). The data is presented as mean ± SD from the analysis of 16 images from two separate culture dishes. Each image showed on average 25 cells. (e–g) Co-localisation analysis of confocal microscopy images (e,f) showing Aβ signal overlap with LysoTracker Deep Red. (g) Mander’s coefficient ± SD for the overlap of Aβ with Lysotracker Deep Red was calculated by analysis of 21 images with ~5 cells in each image (from three separate samples). The cells were incubated for 24 h, washed and incubated for an additional 5 h prior to imaging. (h) Flow cytometry histograms showing the cellular uptake of Aβ(1–40) and Aβ(1–42) following incubation with 1 μM peptide for 5 h. The baseline, representing cellular autofluorescence, is shown for comparison. (i) Uptake of Aβ(1–40) and Aβ(1–42) as function of peptide concentration following 5 h of incubation. (j,k) Uptake of Aβ(1–40) and Aβ(1–42) as function of incubation time at a peptide concentration of 500 nM. (l) Clearance of intracellular Aβ(1–40) and Aβ(1–42). Cells were loaded by incubation with 500 nM peptide for 24 hours (“0 h”), washed and incubated for an additional 24 h (“24 h”). The data has been corrected for cell division. Uptake in (i–l) is reported as mean cellular fluorescence intensity ± SD of the total number of gated live cells in three replicates. Data were corrected for baseline contributions by subtracting the mean cellular autofluorescence.
