Abstract
Unprocessed colored effluents containing dyes are released from several industries resulting in severe water pollution. There was a need to cope with this problem, so an abundant industrial waste of grey stone (GS) powder was utilized for acid yellow dye removal. Scanning electron microscopy (SEM) showed that after heating GS powder at elevated temperatures calcination resulted in the production of nanorods. Optimization of factors to enhance adsorption efficiency was done using different values of factors like pH, initial dye concentration, temperature, adsorbent doses, and contact time. To further increase the yellow dye removal capacity of calcined grey stone (CGS) powder, its nanocomposite was prepared using copper doped titanium dioxide. Most interesting observation recorded during the present study was generation of nanorods on calcination of waste powder which then further attained shape of circular particles on producing composites with copper doped titanium dioxide. The composite exhibited a higher external surface area as confirmed by BET (Brunauer-Emmett-Teller) analysis available for dye removal. SEM micrograph showed that nanocomposite had regular shaped small particles. The study highlighted the potential of using low-cost, sustainable adsorbents for efficient acid-yellow dye removal, addressing the pressing issue of industrial water pollution. These findings provide the directions to combat water pollution to save water resources and for a healthier environment.
Similar content being viewed by others
Introduction
Water and air pollution caused by industries causes severe environmental problems such as resource depletion, and biodiversity loss1. The pollutants released because of industrial activities are responsible for health issues, damaging freshwater resources, causing soil and air pollution. This emphasizes the need for effective treatment methods, and strict pollution control measures to reduce the impacts of industrial wastewater on water quality and human well-being2. So, ensuring the sustainability of our freshwater resources has become an urgent and pressing global need, affecting various aspects of life and the environment3,4. Colored water released from dyeing processes in textile and leather production needs urgent attention for critical environmental protection. The presence of color reduces or stops the penetration of sunlight into wastewater and ultimately disturbs natural environmental remediation processes. The industry’s use of over 8,000 chemicals and massive water consumption, with wastewater containing 72 toxic chemicals, including non-removable ones, poses a severe threat to aquatic ecosystems and human health due to the release of carcinogenic agents and chromosomal deformities. Dyes and pigments are colorants used to enhance the appearance of materials, with synthetic dyes having replaced natural sources due to their stability and wide color range. The textile industry is one of the most water-consuming sectors, produces vast amounts of wastewater contaminated with synthetic dyes, chemicals, and metals, posing a significant environmental threat. Over 100,000 commercial dyes exist, categorized by application and chemical structure, a substantial proportion of textile wastewater results from dyeing, which generates highly saline and biodegradable waste. Dyes vary in their biodegradability, with some being non-biodegradable and toxic, further complicating wastewater treatment. Dye pollution in water bodies, stemming from various industries, poses a significant environmental challenge due to the discharge of synthetic dyes, which can alter water quality and adversely affect aquatic life and human health5.
Efforts to address dye removal have led to the development of various chemicals, physicochemicals, and biological methods. These approaches continue to evolve, and integrated processes show adsorption as a promising approach in effectively treating dye-laden wastewater. Adsorption is a cheap method to remove toxic dyes from wastewater6,7,8. It removes dyes from effluents with great effectiveness by attachment with active sites on adsorbent surface. Photocatalysts such as TiO2 are highly effective in degrading toxic dye pollutants present in wastewater. There is the possibility of coupling adsorption with photocatalysis for dye removal in a cost-effective way for efficient treatment. The present study was undertaken to optimize various process parameters to remove acid yellow dye from wastewater using adsorbents. In addition, a composite material composed of adsorbent and photocatalyst was also utilized to remove acid yellow dye from wastewater.
Materials and methods
Calibration curve Preparation
A series of standard dye solutions were prepared and absorption of solutions was measured to construct calibration curve (Fig. 1). The spectral characteristics of acid yellow dye were investigated using a UV-visible spectrophotometer from 332 to 1000 nm. The experiments were carried out at the dye pH.
Preparation of adsorbents and composite
In exploring sustainable adsorbent materials, this research investigated the potential of GS powder waste. The initial stages encompassed the purification of the GS powder by washing it with distilled water, followed by a drying process. Subsequently, the dried GS powder was ground into a fine powder and sieved to ensure uniformity in particle size, with the target range set at 100 to 200 mesh. A notable aspect of the study involved subjecting a portion of the powder to a thermal treatment, involving heating in a crucible at 300 ˚C for 24 h. After cooling, the material was processed again, and a powder known as calcined GS powder was produced. The Cu doped TiO2 was synthesized via wet impregnation method by suspending 10 g TiO2 nanoparticles in 20 ml of distilled water by slow stirring. Cupric sulphate (0.2 g) were added dropwise and magnetically stirred for 30 min. The produced slurry was dried in an oven (at 60 °C) overnight. Then the dried 2% Cu. TiO2 powder was finally calcined at 450 °C for 2 h9. To prepare CGS-2% Cu.TiO2 composite, equal amounts of 2% Cu. TiO2 and CGS powders were mixed in equal amounts to prepare a slurry. The obtained slurry was heated at 450 °C for 2 h for activation.
Optimization of parameters
In the order to optimize dye adsorption, batch experiments were conducted at various concentrations of dye, and key parameters like pH (5–10), adsorbent dosage (0.005–0.04 g/10 mL), dye concentration (5–50 ppm), temperature (30–70 °C), and contact time (15–240 min). Following each experiment, separation of the adsorbate solution from the adsorbent was achieved through centrifugation. Photocatalysts TiO2 and 2% Cu.TiO2 efficiency to remove acid yellow dye was tested by placing samples in direct sunlight. Filtrate absorbance to determine concentration was measured at 427 nm using a UV–visible spectrophotometer.
The amount of dye adsorbed at equilibrium (qe) (mg/g) was calculated using Eq. (1), where Co and Ce represented initial and equilibrium concentrations of the dye in the liquid phase (mg/L), V denoted the solution volume, and W indicated the adsorbent weight. This approach aimed to enhance understanding and identify optimal conditions for maximum adsorption efficacy10.
Adsorption isotherm
The adsorption isotherms heavily rely on equilibrium studies and describe distribution of molecules between solid and liquid phases. This study used a variety of isotherm models, such as the Freundlich, Dubinin–Radushkevich (D–R), Langmuir, Temkin, and Harkins–Jura models. Freundlich, Dubinin–Radushkevich (D–R), Langmuir, Temkin, and Harkins–Jura models isotherms’ parameters provide an idea about the sorption mechanism, the affinity between adsorbent and molecules being sorbed, and its surface characteristics10.
Adsorption kinetics
To investigate the adsorption kinetics, the adsorbent was mixed with 50ppm dye solution at pH 7, agitated at 200 rpm at room temperature for different time intervals. After centrifugation, the concentration of acid yellow dye at various times was measured using the calibration curve. The influence of temperature on adsorption was determined at different temperatures (30, 40, 50, 60, and 70 °C) for a range of time intervals of 15, 30, 60, 120, and 240 min was investigated10.
Textile industry wastewater
Textile industry wastewater was collected from wastewater channel (Samundri road drain), Faisalabad and was immediately filtered, stored in opaque glass bottle and immediately transported to lab for further experimentation11.
Characterization studies
The SEM images of adsorbents and composites were generated using the Nova NanoSEM 450, FEI, Oregon, USA equipped with built in ETD and TLD. Malvern, Zetasizer Nano ZSP was used to measure the particle size (hydrodynamic diameter) and size distribution. BET analysis was performed using Autosorb BET apparatus from Quantachrome Corporation.
Results and discussion
Optimization of pH
The solution pH is an important parameter in adsorption studies as surface charges, adsorbent surface and dye ionization is dependent on it12,13,14. The effect of pH is presented in Fig. 2 for acid yellow dye removal using GS powder and CGS powder, over varied pH levels (5 to 10). The dye concentration was kept constant at 50 ppm, and the dose was kept constant at 0.005 g/10 mL. The qe percentage dye removal values for both adsorbents changed when the pH rises from 5 to 10. The effect of pH in dye removal is critical and multidimensional, regulating the surface charge of the adsorbent and dye molecules and delicately altering the adsorption process. Adsorption of dye from wastewater is strongly affected by pH which makes pH a critical parameter in dye adsorption studies15. It controls the surface properties, adsorbent’s charge, and adsorbate ionization level. The charge on adsorbent surface is pH based. The adsorbent’s surface charge becomes positive when the pH falls below a certain threshold and negative when the pH rises beyond that threshold. In case of acid yellow dye adsorption using GS and CGS powder, it was found that dye removal decreased after pH 7 as pH increased. The important interaction that occurs between dye and adsorbent molecules are electrostatic forces and hydrogen bonding. In the alkaline media, there might be decreased interaction between adsorbent active sites and dye molecules that resulted in reduced dye adsorption16.
Optimization of dose
The experimental data presented dynamics of dye adsorption onto GS powder and CGS powder, shedding light on crucial parameters influencing the process (Fig. 3). Interestingly, as the dose of GS powder increased, the adsorption capacity exhibited a counterintuitive trend, with higher doses resulting in decreased dye removal. This inverse relationship between dose and adsorption capacity aligns with typical behavior observed in adsorption processes, emphasizing the intricate nature of these interactions. The observed inverse proportionality between sorbent dosage and sorption capacities was observed previously in similar studies emphasizing the need for careful optimization of the adsorbent mass about sorption efficiency17.
Optimization of initial dye concentration
The concentration of the dye varied from 5 to 50 ppm (Fig. 4). The adsorption capacity increased as the concentration of the dye increased. At higher dye concentrations more dye molecules are available to interact with adsorption sies as compared to lower concentrations. But this is true up to saturation limit of adsorbent surface. When initial dye concentration increased, the percentage of dye removal decreased, but the interaction effect was insignificant at lower adsorbent doses18.
Optimization of time
The results of time required for adsorption of acid yellow dye are presented in Fig. 5. Removal of acid yellow dye was studied at varying intervals of 15, 30, 60, 120, 180 and 240 min at 30, 40, 50, 60 and 70 °C while keeping all other parameters constant. At all temperatures, the adsorption of dye occurred in two phases. The first phase involved the rapid adsorption of dye till 60 min followed by the steady phase that occurred to 240 min to attain equilibrium. At the initial stage, the adsorbent material surface was unoccupied and had many free available sites which resulted in a high rate of dye adsorption. As this time proceeds, these adsorption site becomes populated, and the rate of adsorption becomes slow and reaches a steady equilibrium. At equilibrium, the rate of adsorption of dye molecules was equal to the rate of desorption of dye molecules. This suggests contact time is an important parameter in adsorption studies19,20.
Optimization of temperature
Temperature effect on dye adsorption is a complex process and depends upon the chemical nature of dye, adsorbent material and solution conditions. Adsorption process can be generally either endothermic or exothermic which is favored at higher or lower temperatures, respectively21. Figure 6 shows results of optimization of temperature for acid yellow dye from 30 to 70 °C. The results indicated that as temperature increased up to 40 ˚C, the adsorption capacity of the material increased, and more dye removal was observed. Temperature played a distinctive role, with acid yellow dye showing increased adsorption at 40 ˚C than other temperatures, reflecting different molecular behaviors at various temperatures. Generally, the rate of dye molecules diffusion to the adsorbent material surface increases with increasing temperature, but on the other hand, an increase in temperature after a certain limit can also weaken the bonds between the adsorbent and the dye, potentially leading to desorption. Therefore, it is necessary to optimize temperature for adsorption studies. The optimized temperature of acid yellow dye removal during the present study was found to be 40 ˚C.
Adsorption isotherm and kinetics
Isotherms not only help to find out the adsorbent maximum adsorption capacity but are also useful for determining the nature interaction between dye-adsorbent molecules. The parameters of adsorption isotherms and kinetic models22 used during the present study are presented in Tables 1 and 2. To study adsorption isotherms; the experiments were conducted at different dye concentrations. After equilibration, samples were collected and filtered, and their residual dye concentrations were measured. The percentage removal of dye was calculated at equilibrium. Five diverse types of isotherms, Langmuir, Freundlich, DR Harkin Jura, and Temkin, were employed to analyze the data. Choosing the most suitable isotherm model relies on correlation coefficients. The results found values of correlation coefficient closer to one for Freundlich isotherm. In addition, experimental adsorption capacity of acid yellow dye also agreed well with calculated adsorption capacity for Freundlich isotherm. Isotherms parameters are presented in Table 1. The analysis, supported by R2 values close to unity, emphasized the Freundlich isotherm’s suitability for describing dye’s adsorption onto the adsorbents. The Freundlich model further substantiated the robust adsorption capacity of the adsorbents, as indicated by the high values of KF. This all strongly suggested that Freundlich was best for this process. Freundlich adsorption isotherm considers that adsorption occurs on a heterogeneous surface with varying adsorption energies. The pseudo second order kinetic model describes that the rate of adsorption is proportional to the square of the number of unoccupied active sites on the adsorbent surface. In addition, it also assumes the rate-limiting step as a chemical reaction (chemisorption) which act through sharing of valence forces or electrons exchange between the adsorbent and adsorbate. The close agreement between experimental and pseudo second order kinetic model, in addition to its higher value of R2 suggested that it better represented acid yellow adsorption process. Pseudo second order kinetic model fitted well to adsorption data in comparison to pseudo first order model (Table 2)23.
Composite adsorption ability and reusability
TiO2, Cu doped TiO2, activated carbon and CGS-2% Cu.TiO2 composite has been utilized to remove acid yellow dye at optimized conditions (Fig. 7a). Titanium dioxide (TiO2) has a wide number of applications as photocatalyst in environmental studies. Though, nontoxic, environmentally friendly and readily available; lower efficiency, less energy harvesting within the UV–Vis range with the ease of photogenerated charge recombination is still a concern for its full-scale deployment as a photocatalyst for environmental remediation applications. Cu doped TiO2 exhibited better acid yellow adsorption potential as compared to pure TiO2. This is due to higher photo catalytical potential of Cu doped TiO2 due to the reduced band gap and altered electronic structure introduced by Cu doping. Which allowed better utilization of solar energy for efficient removal of acid yellow dye. A better acid yellow removal capacity was shown by activated carbon than TiO2 which was comparable to Cu doped TiO2. The composite materials CGS-2% Cu.TiO2 was composed of CGS powder (an efficient adsorbent) and Cu doped TiO2 (an efficient photocatalytic material). CGS-2% Cu.TiO2 composite not only exhibited better acid yellow dye removal potential than TiO2, and Cu doped TiO2 but also reached to equilibrium in only 60 min as compared to other two materials which took 240 min. Regeneration of adsorbent materials is crucial for environmental sustainability, cost-effectiveness and minimizing the need for frequent adsorbent disposal and replacement24. All materials used for removal of acid yellow dye removal for five successive cycles without any significant loss in dye adsorption capacity (Fig. 7b).
Textile industry wastewater treatment
CGS-2% Cu.TiO2 composite was found to be the most efficient material developed during the present study for dye removal. Colored wastewater sample collected from the textile industry was subjected to dye removal process using CGS-2% Cu.TiO2 composite (Fig. 8). The results showed that dyes adsorption process from the textile industry was very efficient and reached equilibrium at 240 min.
SEM and zeta analysis
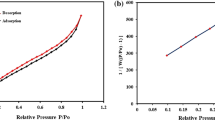
SEM analysis of GS and CGS powder has shown very promising results and clearly suggested that this material can be potentially used for water treatment based on its surface morphology (Fig. 9). SEM micrograph of GS powder shows that it contains large granular particles. On calcination GS powder has attained nanorod structure. The calcination process resulted in elimination of gases and volatile materials from the powder. SEM micrograph of copper doped titanium nanocomposite with CGS powder showed that it has circular shaped nano particles. Zetasizer analysis confirms that CGS powder had mainly two types of particles. It is also confirmed by the presence of two types of peaks. The peak 1 has 461.1 d.nm (size), 93.3% (intensity) and 101.3 (St Dev). The peak 2 has 97.17 d.nm (size), 6.7% (intensity) and 13.30 (St Dev). Z-Average (d.nm) was 680.8. Copper doped titanium dioxide and CGS powder composite has shown peak 1 has 442.9 d.nm (size), 94.6% (intensity) and 165.3 (St Dev). The peak 2 has 5299 d.nm (size), 5.4% (intensity) and 405.0 (St Dev). Z-Average (d.nm) was 507.4. The higher intensity peaks both in CGS powder and CGS-2% Cu.TiO2 composite suggested the aggregation of particles. The aggregation of particles could be useful for easy separation of adsorbent materials from colored solutions. For CGS powder, micropore volume and micropore area were 0.006 cc/g and 11.964 m²/g, respectively. CGS powder external surface area was found to be 1.229 m²/g. For CGS-2% Cu.TiO2 composite, micropore volume was 0.003 cc/g and micropore area was 7.175 m²/g. External surface area for CGS-2% Cu.TiO2 composite was 7.175 m²/g. Higher external surface area of CGS-2% Cu.TiO2 composite clearly represents that it can more easily interact with acid yellow dye molecules. The characterization studies play a vital role in identifying the used material properties25.
Conclusions
Finally, the study addressed the serious environmental issue of water pollution caused by releasing acid yellow dye from industrial effluents. The study explored the possibilities of locally sourced GS powder in its natural and calcined forms as an economical and sustainable adsorbent for efficient acid yellow dye removal to address this issue. The optimized pH was 7. The acid yellow dye removal capacity was high at lower adsorbent dose and higher dye concentrations. Most of the dye was removed during the first 60 min of contact time. However, in the case of textile industry effluent the equilibrium time was 240 min. Acid yellow dye removal was favored at 40 ˚C as compared to other temperatures. CGS-2% Cu.TiO2 composite outperformed other materials used in the present study in acid yellow dye removal. These findings emphasize the significance of optimizing various parameters for optimum adsorption efficacy, opening the way for developing efficient and ecologically friendly water treatment processes.
Data availability
All data generated or analysed during this study are included in this published article.
References
Khan, M., Qadri, A. & Raza, H. Advancements in environmental (water, air, soil) protection technologies: A review. Int. J. Chem. Biochem. Sci. 25, 575–592 (2024).
Mohammed Alsuhaibani, A., Alayyafi, A. A., Albedair, L. A., El-Desouky, M. G. & El-Bindary, A. A. Synthesis and characterization of metal–organic frameworks based on thorium for the effective removal of 2,4-dichlorophenylacetic pesticide from water: batch adsorption and Box-Behnken design optimization, and evaluation of reusability. J. Mol. Liq. 398, 124252. https://doi.org/10.1016/j.molliq.2024.124252 (2024).
Zahra, R. & Jilani, M. I. Improved constructed wetlands for treating water. Int. J. Chem. Biochem. Sci. 25, 211–228 (2024).
Batool, R. & Alam, M. Removal of microplastics and nanoplastics from water: A review. Int. J. Chem. Biochem. Sci. 25, 193–207 (2024).
Adar, E. Removal of acid yellow 17 from textile wastewater by adsorption and heterogeneous persulfate oxidation. Int. J. Env Sci. Technol. 18, 483–498. https://doi.org/10.1007/s13762-020-02986-5 (2021).
Abbas, S. et al. Adsorption of crystal Violet dye by using a low-cost adsorbent – peanut husk. Desalin. Water Treat. 233, 387–398. https://doi.org/10.5004/dwt.2021.27538 (2021).
Çiçek, F., Özer, D., Özer, A. & Özer, A. Low cost removal of reactive dyes using wheat Bran. J. Hazard. Mater. 146, 408–416 (2007).
Sardar, M., Manna, M., Maharana, M. & Sen, S. In Green Adsorbents To Remove metals, Dyes and Boron from Polluted Water 377–403 (Springer, 2020).
De, A. & Boxi, S. S. Application of Cu impregnated TiO2 as a heterogeneous nanocatalyst for the production of biodiesel from palm oil. Fuel 265, 117019 (2020).
Li, K., Wu, J., Li, X., Li, B. & Zhou, D. Preparation of porous composite hydrogel with ultra-high dye adsorption capacity based on biochar: adsorption behaviors and mechanisms. Chem. Eng. Sci. 295, 120115 (2024).
Uddin, F. Environmental hazard in textile dyeing wastewater from local textile industry. Cellulose 28, 10715–10739 (2021).
Crini, G., Peindy, H. N., Gimbert, F. & Robert, C. Removal of CI basic green 4 (Malachite green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep. Purif. Technol. 53, 97–110 (2007).
Javadian, H., Sorkhrodi, F. Z. & Koutenaei, B. B. Experimental investigation on enhancing aqueous cadmium removal via nanostructure composite of modified hexagonal type mesoporous silica with polyaniline/polypyrrole nanoparticles. J. Ind. Eng. Chem. 20, 3678–3688 (2014).
Wang, N., Li, J., Lv, W., Feng, J. & Yan, W. Synthesis of polyaniline/TiO 2 composite with excellent adsorption performance on acid red G. RSC Adv. 5, 21132–21141 (2015).
Aravindhan, S., Kumar, G. B., Saravanan, M. & Arumugam, A. Delonix regia biomass as an eco-friendly biosorbent for effective Alizarin red S textile dye removal: Characterization, kinetics, and isotherm studies. Bioresource Technol. Rep. 25, 101721 (2024).
Sharma, K. et al. Methylene blue dye adsorption from wastewater using Hydroxyapatite/Gold nanocomposite: kinetic and thermodynamics studies. Nanomaterials 11, 1403 (2021).
Ali, N. S., Khader, E. H., Abdulrahman, M. A., Salih, I. K. & Albayati, T. M. Removal of anionic Azo dye from wastewater using Fe3O4 magnetic nanoparticles adsorbents in a batch system. Desalin. Water Treat. 317, 100033 (2024).
Meskel, A. G. et al. Malachite green and methylene blue dye removal using modified Bagasse fly ash: adsorption optimization studies. Environ. Challenges. 14, 100829 (2024).
Nadafi, K., Vosoughi, M., Asadi, A., Borna, M. O. & Shirmardi, M. Reactive red 120 dye removal from aqueous solution by adsorption on nano-alumina. J. Water Chem. Technol. 36, 125–133 (2014).
Hamad, K. H. et al. Nylon fiber waste as a prominent adsorbent for congo red dye removal. Sci. Rep. 14, 1088 (2024).
Soltani, A., Faramarzi, M. & Mousavi Parsa, S. A. A review on adsorbent parameters for removal of dye products from industrial wastewater. Water Qual. Res. J. 56, 181–193 (2021).
Galloni, M. G., Bortolotto, V., Falletta, E. & Bianchi, C. L. pH-Driven selective adsorption of Multi-Dyes solutions by loofah sponge and Polyaniline-Modified loofah sponge. Polymers 14, 4897 (2022).
Gharbani, P. & Mehrizad, A. Preparation and characterization of graphitic carbon nitrides/polyvinylidene fluoride adsorptive membrane modified with Chitosan for Rhodamine B dye removal from water: adsorption isotherms, kinetics and thermodynamics. Carbohydr. Polym. 277, 118860 (2022).
Aljeboree, A. M., Alkaim, A. F., Alsultany, F. H. & Issa, S. K. Highly reusable nano adsorbent based on clay-incorporated hydrogel nanocomposite for cationic dye adsorption. J. Inorg. Organomet. Polym Mater. 35, 1165–1186 (2025).
Mozhiarasi, V. & Natarajan, T. S. Bael fruit shell–derived activated carbon adsorbent: effect of surface charge of activated carbon and type of pollutants for improved adsorption capacity. Biomass Convers. Biorefinery. 14, 8761–8774 (2024).
Author information
Authors and Affiliations
Contributions
Rabia Shaheen; Research work, article writeup, Data interpolation and discussion; Muhammad Asif Hanif; Supervision and manuscript proof-read, Statistical evaluation, Figures, and table preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shaheen, R., Hanif, M.A. Titanium dioxide photocatalyst enabled composite for dye removal from water. Sci Rep 15, 41293 (2025). https://doi.org/10.1038/s41598-025-25138-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-25138-6