Abstract
In this study, the insightful photocatalytic properties and efficiency of TiO2-BiOBr heterojunctions embedded within clinoptilolite matrix for the simultaneous H2 generation and bentazon-polluted water treatment were assessed. The characterization outcomes indicated that immobilizing TiO2-BiOBr heterojunction has the potential to enhance dispersion and arrangement of photoactive components, while mitigating the recombination rate of heterostructure. These characteristics become increasingly prominent as the TiO2/BiOBr weight ratio in the embedded heterogeneous structure rises. This betterment can be originated from the enhancement in the interaction of TiO2 with BiOBr species which elevates likely of creating stronger TiO2-BiOBr heterojunction and inhibits the creation of defects sites and the growth of bismuth-based nanosheets. Moreover, raising the content of immobilized TiO2-rich heterojunction (TiO2/BiOBr weight ratio of 3/1) from 0 to 40 wt.% elevated the elimination efficiency while it declined upon further increasing the immobilization content. Although specific surface area increases, overloading of TiO2-BiOBr heterojunction as a result of the formation of more surface agglomerates causes a significant diminution in the number of accessible photoactive sites and inappropriate interaction between clinoptilolite and TiO2-BiOBr heterojunction which restrict the absorb light and lifetime of electron–hole pairs, respectively. In accordance with characterization results, it was found that embedding 40 wt.% TiO2-rich heterojunction within zeolite matrix endows the best photocatalytic activity towards bentazon elimination. The experimental findings also demonstrate the high effectiveness of this sample in the concurrent photocatalytic processes of hydrogen production and bentazon-contaminated wastewater treatment. Under irradiation of UV and simulated solar lights, the hydrogen generation rate of 3731 μmol.g−1.h−1 along with the complete elimination of bentazon and the rate of hydrogen generation of 2853 μmol.g−1.h−1 with a removal efficiency of 81% were attained, respectively.
Similar content being viewed by others
Introduction
The occurrence of energy crises and environmental challenges constitutes major global challenges that present substantial risks to human development and the maintenance of ecological balance1,2,3. Hence, the substantial strategies for attaining sustainable development encompass the efficient diminution of environmental pollution issues alongside the concurrent advancement of clean, affordable, and renewable energy sources4. Hydrogen has been increasingly accepted as a viable alternative to fossil fuels due to its abundant availability and renewable nature5,6. Currently, hydrogen production is dependent on fossil fuels, which raises significant concerns regarding its sustainability and contributes to carbon emissions7. Unlike conventional methods, photocatalytic water splitting offers a clean and environmentally friendly alternative for hydrogen production. However, the use of water in this process raises concerns about competing demands for this precious resource, particularly in areas already experiencing water scarcity. Addressing this obstacle requires prioritizing sustainable water management strategies and investigating substitute sources of water. Recently, organic pollutants-contained wastewater, traditionally viewed as a burden, has been caught the attention of researchers as a readily available and potentially valuable feedstock for hydrogen production. Agricultural wastewater, originating from the use of various pesticides to boost the crops productivity is one of the major sources of organic contaminants which have caused serious environmental issues. Bentazon as a selective post-emergence herbicide is considered as one of the most common pesticides used in modern agriculture. Despite the controlled application of high-quality bentazon herbicide in agricultural fields, due to its high-water solubility and mobility in soil, these relatively toxic substances can readily leach into surface and groundwater, necessitating an urgent need for their removal from agricultural wastewater8,9,10. Implementing agricultural wastewater within photocatalytic process of hydrogen production not only mitigates environmental impacts and water shortage crisis but also can help minimize water consumption. Indeed, photocatalytic hydrogen production from agricultural wastewater by integrating wastewater treatment principles into photocatalytic water splitting strategy represents a dual benefit of environmental remediation and resource recovery11,12.
TiO2 is considered a highly hopefully candidate for the development of a dual-functional photocatalyst. This is attributed to remarkable photochemical stability, low cost, non-toxicity, and environmentally friendly nature13,14,15,16. However, bare titanium dioxide exhibits several significant limitations, including low adsorption capacity, a limited ability to efficiently separate charge carriers, weak retrieval from aqueous solutions, and insensitivity to visible light13,17,18,19. The employment of heterojunctions between TiO2 and compatible semiconductors presents a promising approach to address the aforementioned limitations, leading to enhanced photo-response, efficient separation of photoexcited electron–hole pairs, and improved photocatalytic performance20. Bismuth oxybromide (BiOBr) is a layered oxide semiconductor that is formed of tetragonal [Bi2O2]2+ slabs enclosed by dyadic Br− layers21,22,23. This compound has garnered significant attention because of its remarkable chemical stability, narrow band gap (Eg = 2.8 eV), and exceptional photocatalytic performance24,25,26,27. Due to the distinct surface characteristics of BiOBr and TiO2, by combining these two semiconductors, a sufficient number of adsorption and reaction sites can be created to effectively carry out various types of photocatalytic processes. Qi et al. synthesized a BiOBr-TiO2 composite material to facilitate the photodegradation process of sodium ethyl xanthate under visible light irradiation. The study revealed that the composite exhibits enhanced adsorption capacity and photocatalytic performance in comparison with individual bismuth oxybromide and titanium dioxide materials. Furthermore, the photocatalytic reaction rate constant for the binary material was found to be significantly higher, with values 2.7 and 40.3 times greater than those of bare BiOBr and TiO2, respectively28. Yu et al. achieved an approximately 92% reduction in the concentration of the specified color pollutant (RhB) through the use of a TiO2-Au-BiOBr photocatalyst29. In other study, a TiO2/BiOBr composite was suggested to enhance the decomposition of methyl orange (MO) and gaseous formaldehyde (HCHO) through photocatalysis. The optimized composite demonstrated decomposition rates of 97% for methyl orange and 60% for formaldehyde. They claimed that the establishment of this configuration notably enhances the separation efficiency and the transfer rate of electron–hole pairs24. The assessment of the photocatalytic efficiency of L-proline-TiO2/BiOBr for the elimination of methylene blue (MB) and Congo red (CR) as specific color contaminants revealed that the incorporation of BiOBr into TiO2 caused a notable increment in the photodecomposition rate through the mitigation of recombination processes. Under optimum conditions, a degradation efficiency of 94% for CR and 83% for MB was recorded30. Fang et al. successfully synthesized a ternary photocatalyst composed of Au, BiOBr and TiO2 with oxygen vacancy, and assessed its efficacy in catalyzing water splitting for hydrogen production. The photocatalyst had exhibited a hydrogen evolution rate of 384 μmol. g-1. h-1 under UV light exposure31. Despite the superior photocatalytic activity demonstrated by BiOBr-TiO2 heterostructure compared to pure TiO2, this particular photocatalyst still faces certain limitations such as rapid accumulation in solution, insufficient recovery capability, and elevated production costs, all of which hinder its practical implementation in the photocatalytic process. Immobilizing BiOBr-TiO2 heterostructure onto porous and high surface area materials can effectively address these challenges. This approach also reduces electron–hole pair recombination and simplifies the separation of the photocatalyst following the reaction, resulting in enhanced photocatalytic efficiency and reusability32,33,34. When the substrate, like zeolite, possesses a persistent internal electric field, it facilitates the dispersion of the charge carriers within the semiconductor material embedded in the zeolite matrix and leads to a greater separation of e-/h+ pairs compared with neutral substrate materials like activated carbon, etc35. Recently, there has been a noteworthy rise in the utilization of natural zeolites as support in the photocatalytic process. This can be attributed to their porous structure, chemical durability, substantial specific surface area, exceptional adsorption characteristics, plentiful availability, and cost-effectiveness36,37. Clinoptilolite, a commonly occurring aluminosilicate mineral, possesses a layered and porous structure and is characterized by an abundance of micropores. It exhibits notable attributes such as high cation-exchange capacity, eco-friendliness, and thermal and chemical stability. Consequently, clinoptilolite is a highly promising support material for heterostructure catalysts13,38,39. Zhou et al. developed a new ternary nanocomposites (BTC) comprising BiOCl, TiO2, and clinoptilolite, resulting in notably enhanced photocatalytic performance under visible light. The remarkable photodegradation efficiency observed in this study can be ascribed to the enhanced synergistic impact achieved by integrating clinoptilolite support with the BiOCl/TiO2 heterojunction within the ternary heterogeneous structure36. More than 90% of sodium isopropyl xanthate (SIPX) was effectively eliminated within 3 h under visible light irradiation using a MoS2/TiO2/Clinoptilolite ternary photocatalyst. Due to employing clinoptilolite support, the nanoparticles were uniformly distributed within the nanocomposite’s structure resulting in a notable enhancement of the photocatalytic efficiency13.
According to the aforementioned literature review, the concurrent employment of TiO2-based heterojunctions and immobilization over a porous substrate such as clinoptilolite represents a highly effective approach for the fabrication of economically viable and long-lasting TiO2-based nanocomposites that exhibit improved photoactivity. It can be confidently asserted that there is a lack of research on the usage of natural zeolite minerals in conjunction with BiOBr-TiO2 heterojunction for photocatalytic purposes. Prior researches have predominantly focused on examining the photocatalytic properties and performance of nanostructured BiOBr-TiO2 heterojunction in the wastewater treatment. However, there is a gap in the literature regarding the immobilization of this heterostructure onto a zeolite matrix for photocatalysis purposes especially wastewater treatment and concurrently H2 production. Moreover, based on our current understanding, the impact of various compositions and embedded contents of BiOBr-TiO2 heterojunction in the zeolite matrix on the photocatalytic characteristics and performance remains unexplored.
In this study, the novel BiOBr-TiO2/Clinoptilolite photocomposites were synthesized using sono-precipitation procedure, and their photocatalytic properties and efficiency in the decomposition of bentazon were evaluated. In this context, the immobilization impact of TiO2-BiOBr heterostructure within the clinoptilolite framework, along with the influence of various compositions and contents of heterostructure integrated into the matrix were assessed in the photodegradation of bentazon. The prepared photocomposites underwent characterization through a range of analytical methods, such as XRD, FESEM, EDX, N2 adsorption–desorption, DRS, and PL analysis. The optimum composition and loading content of TiO2-BiOBr heterostructure embedded within the clinoptilolite matrix were identified, followed by an assessment of the photocatalytic performance of the best photocatalyst through hydrogen photoproduction and concurrent degradation of bentazon. We anticipate that this research has the potential to open up new possibilities for the efficient benefit of solar energy using TiO2-based photocatalysts, thereby enhancing its practicality in the area of environmental management and energy expansion.
Experimental section
Material
Clinoptilolite with a specific surface area of 15.73 m2/g and chemical composition of SiO2: 70.67%, Al2O3: 11.78%, Na2O: 2.74%, K2O: 1.80%, CaO: 1.73%, MgO: 1.14%, Fe2O3: 0.95%, TiO2: 0.185%, SO3: 0.055%, P2O5: 0.010%, MnO: 0.003%, L.O.I: 8.937%, which was used as a support in the present study, was purchased from Miane mine located in East Azerbaijan province, Iran. Bismuth nitrate (Bi(NO3)3.5H2O, Sigma-Aldrich, 98%), potassium bromide (KBr, Merck, 99%), and commercial TiO2 (Degussa P-25, which contains 80% anatase phase and 20% rutile phase, particle size of 25 nm and specific surface area of 50 m2/g) were utilized as BiOBr precursors and titania, respectively. Ethanol (C2H5OH, Merck, 99%) and double distilled water as solvent, and methanol (CH3OH, Merck, 99.8%) as sacrificial agent in photocatalytic tests of hydrogen production were utilized. Bentazon herbicide (C10H12N2O3S, Shandong Binnong Technology Co., 48% SL) was used as the source of agricultural pollutant.
Fabrication of photocatalyst
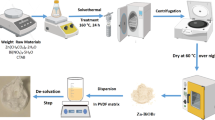
In the current investigation, the immobilization of TiO2-BiOBr photocatalysts onto clinoptilolite structure was achieved through the application of the sonochemically precipitation technique. To prepare the clinoptilolite support, the clinoptilolite ore was initially subjected to grinding and sieving processes. The clinoptilolite powder, which had a particle size ranging from 44 to 53 μm, was subjected to multiple washes with double distilled water in order to eliminate impurities and undesirable substances, and subsequently, it was dried for 24 h at 110 °C prior to its utilization as a support material. In the sonochemically precipitation technique, a uniform solution with a predetermined concentration of bismuth salt was prepared by dissolving the desired quantity of Bi(NO3)3.5H2O in ethanol while subjecting it to agitate for 30 min. Following that, particular quantities of clinoptilolite support and titanium dioxide were introduced into the solution containing Bi(NO3)3.5H2O while being agitated, resulting in the formation of a suspension referred to as suspension A. Concurrently, a separate solution was prepared using potassium bromide (KBr) precursor dissolved in water solvent at a specified concentration, and this solution was designated as solution B. Solution B was gradually introduced into suspension A while maintaining constant stirring. The obtained suspension was sonicated using an ultrasound bath operating at a frequency of 50 Hz and a temperature of 45 °C for 1 h. The obtained precipitates were subjected to an aging process using a reflux system, maintained at a temperature range of 80–85 °C for 4 h. After the completion of the aging process, the resulting precipitates were filtrated and washed using double distilled water multiple, and subsequently, the obtained powders were dried at 80 °C for 12 h, and ultimately, calcined at 350 °C for 3 h. The photocatalytic heterostructures derived from the decoration of clinoptilolite microparticles with TiO2 nanoparticles and/or BiOBr nanosheets were designated as BxTy/CLT, in which the "x" and "y" demonstrators the nominal weight percentage of BiOBr and TiO2 in photocatalyst structure, respectively. The details of the preparation of clinoptilolite support and the synthesis of photocatalysts are shown in Fig. 1.
Photocatalyst characterization
The crystal structure and microstructure of the prepared samples were analyzed using X-ray diffraction analysis. This analysis was conducted utilizing the X´PertPro device of the Philips company, made in the Netherlands, using Cu-kα radiation in the 2θ range between 5 and 80 ° and with a scanning speed of 0.05°/s. The morphology of the particles and surface composition of the synthesized samples were studied by employing the scanning electron microscopy (FEI ESEM Quanta 200, USA) equipped energy dispersive X-Ray spectroscopy (EDAX SILICON DRIFT 2017). The surface porosity, specific surface area, and other textural properties of photocatalysts were examined through N2 adsorption–desorption analysis (BELSORP-mini II, Japan) and the BET and BJH methods. A DRS analysis was conducted utilizing a UV–visible spectrophotometer model V-760 manufactured in Japan to determine the absorption wavelength and bandgap of the synthesized samples. The recombination rate of electron–hole pairs in the synthesized samples was evaluated using photoluminescence spectroscopy (PL) analysis (Avaspec 2048 TEC, Netherlands). This analysis was conducted at an excitation wavelength of 320 nm.
Photocatalytic experiments of hydrogen production and bentazon degradation
A schematic illustrating the experimental configuration employed to assess the efficacy of the prepared photocatalysts is presented in Fig. 2. The photocatalytic production of hydrogen and purification of agricultural wastewater were conducted within a cylindrical quartz reactor, positioned on a magnetic stirrer, and enclosed within an aluminum-covered frame. In order to maintain a consistent temperature range of 10–15 °C within the photoreactor solution, a condenser connected to an ice-water bath was installed within the photoreactor cell. Three 125 W medium-pressure mercury lamps and a 400 W halogen lamp were employed as sources of UV and solar simulator lights, respectively. To facilitate the regulation of temperature within the system and ensure the provision of air conditioning, a ventilator was integrated into the upper section of the frame. To conduct the performance experiment, a quantity of 200 mg of synthesized photocatalyst was evenly distributed within a 200 mL pre-made bentazon solution (20 ppm) within the photoreactor. After sealing the photoreactor, the reaction mixture was deaerated for 15 min using argon gas. Following this, the mixture was magnetically stirred in a dark environment for 2 h to gain adsorption–desorption equilibrium. Subsequently, the light sources were switched on, initiating the photocatalytic process. The quantity of generated hydrogen was measured utilizing a gas chromatography instrument (GC-2552 TG, Iran) featuring a thermal conductivity detector (TCD) and a molecular sieve 5°A column. Furthermore, in order to determine the concentration of bentazon, periodic sampling was conducted. The photocatalyst nanoparticles were subsequently separated by centrifugation for 20 min at a rotational speed of 4000 rpm. Ultimately, the bentazon concentration was determined utilizing a spectrophotometer (T80+, pg instruments) at a specific wavelength of 330 nm.
Results and discussions
Photocatalyst characterization results
XRD analysis
The X-ray diffraction (XRD) patterns depicted in Fig. 3 correspond to the bare clinoptilolite, binary and ternary photocomposites. The examination of the XRD patterns indicates the attendance of distinct peaks associated with TiO2, BiOBr, and clinoptilolite crystalline phases in the samples containing these compounds. There are no discernible diffraction peaks corresponding to any other crystalline phases present in the patterns, thus providing confirmation of the successful preparation of the photocatalysts. The peaks observed at 9.9, 10.9, 22.3, 25.2, 26.1, 28.2, 30.0, and 31.7° are indicative of the monoclinic phase of clinoptilolite, as identified by the JCPDS number of 00–025-134940,41. Furthermore, the observed peaks at angles of 10.9, 25.2, 31.7, 32.2, 34.1, 39.3, 46.8, 50.7, 53.4, 56.1, and 57.1°, corresponding to the crystallographic planes of (001), (101), (102), (110), (111), (112), (113), (104), (211), (114), and (212), respectively, are indicative of the formation of BiOBr tetragonal phase (JCPDS No.: 00–009-0393)27,42,43. The identification of the TiO2 anatase phase (JCPDS No.: 00–001-0562) is substantiated by the observed peaks at 25.2, 37.9, 48.0, 53.9, 55.7, 62.7, 68.9, 70.2, and 75.2°44,45. Identifying the rutile phase is challenging because its characteristic peaks overlap with those of other crystalline phases. Based on the XRD patterns, it can be observed that the intensity of the clinoptilolite peaks in the embedded samples has reduced in comparison to the raw zeolite. This decrease is notably more prominent in the B40T0/CLT sample, possibly attributed to the existence of expanded BiOBr nanosheets and thereupon, greater surface coverage of clinoptilolite relative to TiO2 nanoparticles. The XRD findings of the embedded BiOBr samples demonstrate that a progressive augmentation in the incorporated TiO2 content results in a notable reduction in the BiOBr crystallinity. It is clear that the reduction in the quantity of BiOBr leads to a decline in its crystallinity. Moreover, the increased existence of TiO2, which effectively inhibits the growth of nanosheets, can be another reason. Notably in the B10T30/CLT sample due to the less decoration of the support with bismuth oxybromide and versus the increased incorporation of TiO2 in the photocomposite structure, the BiOBr preeminent peaks are not evident. After a thorough analysis of the XRD patterns of the ternary samples, it is apparent that the intensity of the clinoptilolite peaks is more reduced in the B10T30/CLT sample. It is noteworthy that the quantity of clinoptilolite and embedded heterojunction utilized in all samples remains consistent, while only the composition of the TiO2-BiOBr heterojunction varies. This reduction in the intensity of the zeolite support peak signifies a highly appropriate decoration of the support with semiconductors. In spite of greater content of TiO2 and BiOBr, the crystallinity of the pure BiOBr-TiO2 heterojunction is lower compared to that of the immobilized BiOBr-rich ones which can be attributed to overlapping of BiOBr nanosheets with each other and/or with TiO2 nanoparticles during the precipitation process. Due to the size and morphology of BiOBr particles, they cover a significant number of TiO2 nanoparticles completely and thereupon, the decrease in the TiO2 crystallinity is more evident. The incorporation of BiOBr-TiO2 heterojunction into clinoptilolite matrix enhances the semiconductors’ dispersion and hinders their agglomeration and overlapping. Accordingly, it can be concluded that incorporated BiOBr-TiO2 heterojunctions exhibit better surface dispersion and lower agglomerations, as will be confirmed by EDX dot mappings and FESEM results.
FESEM analysis
Figure 4 presents the images obtained through FESEM analysis for the pure BiOBr sample, as well as the binary and ternary samples. The structure of pure BiOBr exhibits a compact arrangement of nanosheets, consistent with the structure described in the literature46,47. The smooth edges and polygonal structure of the nanosheets confirm the existence of a well-defined crystalline structure and successful crystallization of BiOBr nanosheets48. The nanosheet morphology enables the active phase of BiOBr to possess a greater surface area available for photoreactions, thereby markedly improving the efficiency of the photocatalytic process. By adorning the clinoptilolite microparticles with TiO2 nanoparticles and BiOBr nanosheets, the separation process of semiconductors is effectively facilitated, thereby enhancing the recyclability of the photocatalyst. During the deposition process, the nanomaterials adhere to the microparticles of the support, either as singular particles or clusters and the nanostructures have maintained their nanoparticle and nanosheet configurations subsequent to immobilization on the substrate. The surface coating of the zeolite support masks its blade-like structure, making it undetectable. The FESEM images of the BiOBr-TiO2 sample depict a significant accumulation of nanoparticles and nanosheets. The presence of these accumulations leads to a decrease in the species’ interaction and thereupon, the photocatalytic performance of the sample. It is clearly seen that the ternary samples exhibit a lower occurrence of clustered nanoparticles and nanosheets in comparison to the BiOBr-TiO2 sample. This observation validates the suitable and evenly distributed arrangement of the active phases on the zeolite matrix. These findings highlight the positive impact of the presence of clinoptilolite as a support and the deposition of a heterojunction structure on the uniformity of the morphology of the ternary sample. Evidently, as the weight percentage of TiO2 in the samples rises, the images depict a corresponding increase in the quantity of spherical nanoparticles while a decrease in the number of nanosheets observed in the images. As evident from the analysis, the B10T30/CLT sample exhibits a more uniform morphology with reduced accumulation, indicating stronger interaction between the BiOBr-TiO2 heterojunction and zeolite matrix.
EDX analysis
To determine the elemental composition and distribution on the surface of the photocatalyst, EDX analysis was used, and the findings are illustrated in Fig. 5 and Table. 1. The EDX spectra declare the co-existence of Ti, Bi, Br and O elements as well as the main constituent elements of clinoptilolite (Si, Al, Mg, Fe, K, Ca and Na) in all samples containing these specific elements. With meticulous consideration of the elemental composition findings, it is evident that the constituent elements in the samples’ structure align with the content used during the synthesis and/or fall within the same range. The results, in conjunction with the XRD analysis findings, provide evidence of the precise synthesis of the samples. In addition to the morphological characteristics of the samples, the comparison of elemental mapping images of bare and embedded BiOBr-TiO2 heterojunctions clearly demonstrated the more appropriate dispersion of active phases of BiOBr and TiO2 in the embedded BiOBr-TiO2 samples. This betterment which can be ascribed to the utilization of zeolite matrix is consistent with the conclusions drawn from the FESEM analysis. The comparison of the elemental dot-mapping images of the ternary samples clearly demonstrates that the sample containing 30 wt.% of TiO2 and 10 wt.% of BiOBr exhibits a more favorable surface distribution of semiconductor elements. From another perspective, the overlapping of elements-related dots has reached a minimum level due to the homogeneous distribution of the semiconductors. This advantageous dispersion results in an increase in accessible photosites and somehow stronger interaction between clinoptilolite and the deposited semiconductors which ultimately enhances the photoactivity of the nanostructured composites.
N2 adsorption–desorption analysis
The textural characteristics of photocomposites were examined using N2 adsorption–desorption analysis and the BET and BJH methods, and the findings are presented in Fig. 6. Regarding the results obtained for the distribution of pore diameter size, it can be concluded that the synthesized photocomposites exhibit a mesoporous structure. The formation of a mesoporous structure can alleviate the limitation of internal diffusion and facilitate the accessibility to internal photoactive sites. The assessment of textural quantity depicts that the pore diameter declines with the augmentation of the titanium dioxide content and in contrast to the diminution in the loading of bismuth oxybromide in the samples. The decrease in the pore diameter can be interrelated to the alleviation in BiOBr loading because this semiconductor, as sighted in the FESEM analysis, has a sheet-like structure and slit-like pores, which leads to an increase in the pore diameter. The N2 adsorption–desorption isotherm diagrams conform to the IUPAC classification of type IV and exhibit hysteresis loop type H3. The presence of an H3-type hysteresis loop provides further evidence of the mesoporous structure within the samples. The B0T40/CLT sample exhibits a greater surface area when compared to B40T0/CLT sample. This can be ascribed to the higher surface area and lower support coverage capability of loaded titanium dioxide nanoparticles compared with BiOBr nanosheets. Although the FESEM analysis revealed a notable accumulation of nanoscale particles in the BiOBr-TiO2 sample, however, it is important to acknowledge that this sample lacks support, resulting in a larger surface area in comparison to the ternary samples. Among the ternary photocomposites, the B10T30/CLT photocatalyst exhibits the highest surface area, measuring 22.98 m2/g. There are two arguments supporting this augmented surface area, firstly the content of incorporated TiO2 in the intended sample is higher than other samples, and because of the greater surface area of TiO2 compared with BiOBr, it has caused an increment in the photocomposite surface area. Secondly, the obvious increment in specific surface area can be ascribed to a more uniform structure, reduced formation of aggregates, proper dispersion of semiconductors onto the mineral support, a highly effective interaction between semiconductor species and clinoptilolite, and a smaller particles size. This interpretation, which aligns with the findings of FESEM and EDX analyses, is deemed a plausible explanation for the observed augmentation in surface area. Based on careful consideration of the findings from this analysis, it is anticipated that the B10T30/CLT photocomposite will demonstrate superior photoperformance.
DRS analysis
The DRS absorption spectra of the synthesized samples are revealed in Fig. 7a. The samples exhibit absorption edge within the wavelength range of 420–450 nm. This finding supports the notion that these composites possess photoactivity under visible light. The absorption spectrum of the embedded BiOBr-TiO2 samples demonstrates a shift in the absorption edge towards shorter wavelengths, in contrast to the bare one. This blue shift can be ascribed to the quantization effect, which explains the reduction in particle size and the nanoscale agglomerations during the process of their immobilization onto the clinoptilolite framework. Among the ternary samples, the B10T30/CLT sample exhibits more blue-shifted absorption edge and higher photo-absorption capacity. This could be attributed to more accessible photoactive sites as a result of the decrease in the agglomeration, uniform distribution, and effective interaction between the heterojunction and clinoptilolite framework, which is consistent with the observations from the FESEM and EDX analyses. The band gap energy (Eg) of the photocatalysts was assessed using the Tauc and Kubelka–Munk methods49 and the resultant findings are depicted in Fig. 7b. The band gap content of B40T0/CLT, BiOBr-TiO2, B30T10/CLT, B20T20/CLT, B10T30/CLT, and B0T40/CLT photocatalysts was measured as 2.7, 2.9, 2.85, 2.9, 2.9 and 2.9 eV, respectively. It can be found that by creating a heterojunction and embedding in the zeolite matrix, the band gap is reduced, increasing the photoactivity of the photocatalyst. It can be stated that the alteration in the embedded heterostructure composition does not substantially impact the photoabsorption properties of the ternary photocomposite.
Understanding the potential positions of band edges is essential for analyzing the charge transfer in heterojunction structures and identification of the possible reactive species. The position of the band edge of the photo-heterostructure was ascertained by applying the equations as follows:
The variable X represents the absolute electronegativity of the semiconductor, with specific values of 5.81 eV for TiO2 and 6.18 eV for BiOBr as indicated in the sources cited20,22. The symbol Ee represents the energy level of unbound electrons relative to the hydrogen scale, which is nearly 4.5 eV. On the other hand, Eg indicates the band gap of the pure semiconductors, with specific values of 3.2 eV for titanium dioxide and 2.8 eV for bismuth oxybromide (BiOBr). By replacing the values of X, Eg, and Ee in Eqs. (1) and (2), the determined potentials of the VB and CB for TiO2 are 2.91 and -0.29 V relative to the normal hydrogen electrode (NHE), while for BiOBr, they are 3.08 and 0.28 V relative to NHE, respectively, as illustrated in Fig. 8. The CB potential of TiO2 is more negative (− 0.29 V vs. NHE) than that of BiOBr (0.28 V vs. NHE). This potential difference facilitates the transfer of photoexcited electrons from the CB of TiO2 to the CB of BiOBr. Simultaneously, the VB of BiOBr (3.08 V vs. NHE) is more positive than that of TiO2 (2.91 V vs. NHE), which drives the migration of photogenerated holes from the VB of BiOBr to that of TiO2. This process leads to the formation of a type II heterojunction with a staggered band gap alignment, a phenomenon that has also been documented in previous studies28,50,51. As a result, holes are gathered on TiO2 entities to facilitate oxidation reactions, while electrons are accumulated on BiOBr entities to enable reduction reactions. The migration of energized electron–hole pairs across a type II TiO2-BiOBr interface results in improved charge separation, reduced electron–hole pair recombination rate, and extended longevity of charge carriers. On the other side, clinoptilolite has the potential to serve as a trap for charge carriers, thereby aiding in the prevention of recombination of pairs. Moreover, the use of clinoptilolite as a supporting substrate has the capability to distribute photoactive sites more evenly and amplify light absorption. Hence, it seems that the synergic impact of clinoptilolite and the BiOBr-TiO2 type-II heterojunction offers diverse and beneficial routes for facilitating the transfer of photocarriers, thereby improving the decomposition of bentazon and hydrogen generation during photocatalytic reactions.
The VB energy levels of TiO2 and BiOBr within the hetero-photocatalysts exhibit greater potential compared to the standard potential required for the conversion of H2O/●OH (+ 2.27 V vs NHE) and OH–/●OH (+ 1.99 V vs NHE). Consequently, the presence of these higher energy levels enables the oxidation of H2O molecules and OH– ions by the holes, resulting in the formation of ●OH. The created holes and ●OH have the ability to engage in chemical reactions with the adsorbed pollutants present on the surface of the photocatalyst, causing the degradation of the contaminant molecules. Moreover, when taking into account the reduction potential of O2/●O2–, which is -0.33 V vs. NHE, it is observed that the electrons present in the CB of TiO2 and BiOBr are unable to catalyze the reduction of O2 to ●O2-. However, light irradiation of the employed sources can cause this reaction to occur. Under UV irradiation with a maximum wavelength of approximately 254 nm (corresponding to an energy of 4.88 eV), the available electrons on the valence bands of TiO₂ and BiOBr can be excited to energy levels of − 1.97 eV and − 1.80 eV, respectively, whereas under solar simulator irradiation with a minimum wavelength of 315 nm (corresponding to an energy of 3.93 eV), the electrons in the valence bands of TiO2 and BiOBr can be migrated to the highest available level with an energy equivalent to − 1.02 eV and − 0.85 eV, respectively. Therefore, electrons excited to these energy levels are capable of reducing adsorbed oxygen molecules on the photocatalyst surface, thereby generating superoxide (●O2–) radicals. From another perspective, electrons situated at energy levels lower than 0.7 V can catalyze the conversion of adsorbed O2 molecules into H2O2 through the O2 + 2e– + 2H+ → H2O2 reaction, given the standard potentials of the O2/H2O2 pair being 0.7 V. Based on the information provided, the reactive species identified as ●OH, ●O2–, H2O2, and holes have the capability to interact with the bentazon herbicide molecules, leading to their degradation or transformation into compounds that are no/less harmful to the environment.
PL analysis
Photoluminescence (PL) emission analysis is employed for the assessment of photocarriers recombination rate in semiconductor materials. The diminished intensity of the peaks observed in this analysis signifies a decrease in the rate at which charge carriers recombine. Figure 9 presents the PL spectra of the samples when excited with a wavelength of 320 nm. The PL peak of the B0T40/CLT, B40T0/CLT, and ternary samples is observed at approximately 320 nm. However, the PL signal of the BiOBr-TiO2 sample is detected at about 305 nm. There is a clear and observable decrease in the intensity of PL signal of the clinoptilolite-supported samples especially TiO2-containing ones when compared to that of the binary BiOBr-TiO2 heterostructure. This decline can be ascribed to the migration of electron–hole pairs generated towards the zeolite matrix which acts as an electron trap, effectively inhibiting their recombination. Among the immobilized photocatalysts, the B40T0/CLT sample endows the highest charge carrier recombination tendency. Considering the way the BiOBr nanosheets are placed over clinoptilolite microparticles which weakens their interaction with zeolite matrix, this finding was predictable. By loading TiO2 nanoparticles and the fabrication of BiOBr-TiO2 heterojunction, a decrease in the intensity of PL signal can be found. The creation of an electric field through the heterojunction structure between titanium dioxide nanoparticles and bismuth oxybromide nanosheets facilitates the movement of charge carriers and enhances their separation rate50,52,53. By gradually raising TiO2 content in the embedded BiOBr-TiO2 heterojunction, a reduction in the intensity of PL signal can be found. This betterment can be originated from the enhancement in the interaction with BiOBr which elevates likely of creating BiOBr-TiO2 heterojunction and inhibits the creation of defects in the oxidation sites of heterostructures that act as trapping centers. Additionally, less growth of BiOBr nanosheets and more homogeneous dispersion of deposited semiconductors can be other reasons. Uniform distribution helps to reduce agglomerations and structure defects, which in turn minimizes recombination positions. Hence, it can be concluded that the B10T30/CLT photocomposite represents the highest charge carrier separation efficiency.
Photocatalysis study results
In order to assess the photocatalytic effectiveness of the clinoptilolite-embedded TiO2-BiOBr heterojunctions, a two-stage procedure was performed. Initially, the photocatalytic performance of the samples with different BiOBr/TiO2 weight ratios (3/1, 1/1 and 1/3) and the embedded BiOBr-TiO2 contents (30, 40, 50 and 100 wt.%) were evaluated in the bentazon elimination under UV light to find the optimal composition and content of the embedded BiOBr-TiO2 heterostructure. Thereafter, the best sample was catalytically examined in the photocatalytic H2 production from pure and bentazon containing water in the presence of UV and simulated solar lights.
Bentazon photocatalytic elimination
The impact of BiOBr/TiO2 weight ratio and the content of the embedded BiOBr-TiO2 heterojunction were assessed by examining the efficacy of the synthesized binary and ternary nanocomposites in the photodecomposition of bentazon, as the target agricultural pollutant, under UV light irradiation and the obtained results are presented in Figs. 10 and 11, respectively. It is important to highlight that no photodegradation of bentazon was occurred in the absence of a photocatalyst and photolysis didn’t take place. As viewed in Fig. 10, the photodegradation of the agricultural pollutant is greatly influenced by the variation in the heterostructure composition. With the gradual increase in the TiO2 content, an upward trend in the photodecomposition of the target pollutant is observed which can be related to the corresponding increase in the surface area of the photocomposites, better surface dispersion of BiOBr-TiO2 heterojunction, and lower recombination rate of charge pairs, as previously confirmed in the characterization analyses. These features which are more prominent in the B10T30/CLT photocomposite, cause a corresponding increment in adsorption capacity, the number of accessible photoactive sites and longer lifetime of charge pairs, leading to the improved efficiency of removing the target pollutant. Accordingly, it is evident that clinoptilolite decorated with 10 wt.% of BiOBr nanosheets and 30 wt.% of TiO2 nanoparticles (B10T30/CLT) demonstrates the optimal photocatalytic performance. Using B10T30/CLT photocomposite, 80% of the target pollutant has been photodegraded under 2 h of UV light, which has increased by 2, 2, and 2.5 times in comparison with the removal efficiency of bentazon over B0T40/CLT, B40T0/CLT, and BiOBr-TiO2 samples, respectively.
As inferred from Fig. 11, the embedded content of the BiOBr-TiO2 heterojunction in clinoptilolite matrix plays an important role in the bentazon elimination. Obviously, the application of bare zeolite under light irradiation didn’t lead to any notable alterations in the concentration of agricultural contaminant revealing the main role of BiOBr-TiO2 heterojunction in the photocatalytic treatment of the wastewater. Raising the embedded content of BiOBr-TiO2 heterojunction from 0 to 40 wt.% results in an enhancement in elimination yield. However, this efficiency diminishes with additional increases in the content of the immobilized semiconductors. The highest photocatalytic performance is observed in the sample containing 40 wt.% of the embedded heterojunction. Although specific surface area increases, overloading of BiOBr-TiO2 heterojunction as a result of the formation of more surface agglomerates results in a significantly reduction of accessible active sites and inappropriate interaction between clinoptilolite and BiOBr-TiO2 heterojunction which restrict the light absorption and lifetime of charge carries, respectively. As depicted in Fig. 11 and as predicted based on the PL, FESEM and EDX analyses, the BiOBr-TiO2 sample exhibits the lowest photoactivity in comparison to the other ternary samples. The heterojunction construction between BiOBr and TiO2 semiconductors, along with the adornment of the clinoptilolite support with this heterostructure, leads to a significant increment in the photoremoval yield of the bentazon herbicide in the ternary samples. Apparently, 40 wt.% is the optimal content of heterojunction incorporation into the zeolite matrix.
Simultaneous photocatalytic hydrogen production and bentazon elimination
The performance evaluation of the selected nanocomposite in photocatalytic hydrogen production and simultaneous treatment of agricultural wastewater was conducted, considering the impact of agricultural contaminant and light source on the quantity of hydrogen generated. The results obtained for hydrogen production and bentazon elimination simultaneously are depicted in Fig. 12. As demonstrated, a total of 3731 μmol.g−1.h−1 hydrogen was produced and a complete removal of the bentazon pollutant from the contaminated water was achieved over the B10T30/CLT photocomposite after exposure to 4 h of ultraviolet light. For the purpose of comparison, the hydrogen production capabilities of the optimal photocatalyst were examined via water splitting, utilizing methanol as a traditional sacrificial agent. As depicted in Fig. 12, the utilization of agricultural wastewater as a feed, in comparison to pure water with a 10% (v/v) of methanol, results in a inconsiderable reduction in the quantity of hydrogen generated. Accordingly, and taking into account the environmental and economic benefits, photocatalytic bentazon-contaminated water splitting seems to be promising and practical option towards hydrogen production. Like methanol, bentazon seems to serve as a sacrificial agent and addresses the swift recombination of photo-charge carriers and the potent surface back reaction (SBR) as the challenges associated with hydrogen production through the photocatalytic decomposition of water. Moreover, the chosen photocatalyst demonstrates remarkable efficiency in simultaneous hydrogen production and bentazon degradation under the simulated solar light. This outcome is consistent with the predictions made using the results obtained from the DRS analysis. Under the exposure to simulated sunlight for 4 h, a total of 2853 μmol.g−1.h−1 hydrogen was achieved, and remarkable degradation of 81% of agricultural pollutants was observed.
Comparison of the selected photocatalyst with existing literature sources for benchmarking purposes
To assess the effectiveness of the B10T30/CLT photocatalyst and confirm its photocatalytic activity, its performance in degradation of bentazon pollutant and hydrogen production processes was compared to other bismuth-based compounds mentioned in previous studies. The outcomes of this comparison are presented in Table. 2, which includes the amount of hydrogen produced as well as the efficiency of organic pollutant removal. Based on the findings from studying the performance of various photocatalysts, it is evident that the current sample exhibits exceptional efficiency in both hydrogen production and the treatment of organic containing wastewater. As evident, the quantity of hydrogen production and the bentazon elimination of B10T30/CLT photocatalyst are comparable to and/or greater than those of other bismuth-based photocomposites. It is important to note that the photoactive materials constitutes only 40 wt.% of our sample, with the remaining majority (60 wt.%) being the natural mineral clinoptilolite. The use of clinoptilolite contributes to a reduction in production costs of the photocomposite, making it a cost-effective choice. Given the arguments presented, the results obtained in the current investigation possess heightened importance and have the potential to be effective.
Conclusion
The main outcomes of this research are explained in the subsequent points. First, it appears that embedding a TiO2-BiOBr heterostructure in a natural zeolite matrix is a viable strategy for enhancing its efficacy in photocatalytic applications. The optical and morphological characteristics of the heterostructure are notably improved, leading to an increased presence of photoactive components and delayed recombination of optical carriers. Second, the effectiveness of ternary photocatalysts is depended on the heterostructure weight ratio imobilized over clinoptilolite. It is important to highlight that as the content of TiO2 increases gradually, an upward trend is observed in the photodegradation of bentazone pollutant, and the highest performance was achieved for the photocatalyst comprising 30 wt.% TiO2. At a weight ratio of BiOBr/TiO2:1/3, the distribution of active phases is heightened, resulting in a greater number of photo-sites accessible to bentazon molecules, which ultimately increases the performance. Third, aside from the heterojunction composition, the efficacy of photoremoval is significantly influenced by the amount of clinoptilolite decoration with heterostructure. Raising the quantity of embedded BiOBr-TiO2 heterostructure from 0 to 40 wt.% results in enhanced photoefficacy for bentazon removal. However, further augmentation of clinoptilolite decoration diminishes efficiency due to the obstruction of photo-active sites, surface coverage, and the formation of aggregates. According to the findings, it appears that the photocatalyst with 40 wt.% of embedded heterostructure exhibited maximum removal rate of agricultural pollutants efficacy. Finally, the efficacy of the photocatalyst with the optimal TiO2/BiOBr weight ratio and embedded BiOBr-TiO2 content (B10T30/CLT), in the concurrent processes of hydrogen photoproduction and bentazon photodegradation was assessed and the photocatalyst demonstrated satisfactory performance, yielding 3731 μmol. g−1. h−1 of hydrogen and achieving complete elimination of bentazon under UV radiation. Moreover, the B10T30/CLT photocatalyst exhibits adequate reactivity when exposed to simulated solar light, rendering it a viable choice for various environmental uses, including energy generation and photocatalytic treatment processes.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Fan, Y., Yang, R., Zhu, R. & Zhu, Z. CdS decorated artificial leaf BiVO4/InVO4 for photocatalytic hydrogen production and simultaneous degradation of biological hydrogen production wastewater. Catal. Today 364, 190–195 (2021).
Hao, P. et al. Rational design of CdS/BiOCl S-scheme heterojunction for effective boosting piezocatalytic H2 evolution and pollutants degradation performances. J. Colloid Interface Sci. 639, 343–354 (2023).
Wang, J. et al. An anti-symmetric dual (ASD) Z-scheme photocatalytic system:(ZnIn2S4/Er3+: Y3Al5O12@ ZnTiO3/CaIn2S4) for organic pollutants degradation with simultaneous hydrogen evolution. Int. J. Hydrog. Energy 44, 6592–6607 (2019).
Wang, K. et al. 0D/3D Bi3TaO7/ZnIn2S4 heterojunction photocatalyst towards degradation of antibiotics coupled with simultaneous H2 evolution: In situ irradiated XPS investigation and S-scheme mechanism insight. Appl. Surf. Sci. 596, 153444 (2022).
Rafique, M. et al. Hydrogen Production Using TiO2-Based Photocatalysts: A Comprehensive Review. ACS Omega 8, 25640–25648 (2023).
Raja, A., Son, N., Swaminathan, M. & Kang, M. Synthesis of a powerful single copper/tungsten atom oxide photocatalyst dispersed on the surface of a reduced graphene oxide-titanium composite for H2 production and pollutant degradation. Chem. Eng. J. 468, 143740 (2023).
Behera, U. S., Purohit, B. K. & Byun, H.-S. A comprehensive review of fossil-based hydrogen production: Technological integrations, environmental sustainability, and economic viability. Int. J. Hydrogen Energy 140, 627–652 (2025).
Mir, N. A., Haque, M., Khan, A., Muneer, M. & Vijayalakshmi, S. Photocatalytic degradation of herbicide Bentazone in aqueous suspension of TiO2: mineralization, identification of intermediates and reaction pathways. Environ. Technol. 35, 407–415 (2014).
Amini, A., Rahmani, F., Kkamforoush, M. & Sene, R. A. Bentonite nanoparticles-incorporated ZnO nanofiber mats assembly by electro-centrifuge spinning for efficient photo-degradation of bentazon herbicide: Tuning composition and process optimization. J. Clean. Prod. 414, 137652 (2023).
Berberidou, C. et al. Study of the decomposition and detoxification of the herbicide bentazon by heterogeneous photocatalysis: Kinetics, intermediates and transformation pathways. Appl. Catal. B 200, 150–163 (2017).
Li, G. et al. Sulphur vacancies-VS2@ C3N4 drived by in situ supramolecular self-assembly for synergistic photocatalytic degradation of real wastewater and H2 production: Vacancies taming interfacial compact heterojunction and carriers transfer. Chem. Eng. J. 433, 134505 (2022).
Yang, R. et al. Highly efficient photocatalytic hydrogen evolution and simultaneous formaldehyde degradation over Z-scheme ZnIn2S4-NiO/BiVO4 hierarchical heterojunction under visible light irradiation. Chem. Eng. J. 423, 130164 (2021).
Zhou, P. et al. Hydrothermal synthesis of novel ternary hierarchical MoS2/TiO2/clinoptilolite nanocomposites with remarkably enhanced visible light response towards xanthates. Appl. Surf. Sci. 542, 148578 (2021).
Hu, X., Li, C., Sun, Z., Song, J. & Zheng, S. Enhanced photocatalytic removal of indoor formaldehyde by ternary heterogeneous BiOCl/TiO2/sepiolite composite under solar and visible light. Build. Environ. 168, 106481 (2020).
Bhom, F. & Isa, Y. M. Photocatalytic hydrogen production using TiO2-based catalysts: A review. Global Chall. 8, 2400134 (2024).
Peng, C. et al. Regulation of the rutile/anatase TiO2 phase junction in-situ grown on–OH terminated Ti3C2Tx (MXene) towards remarkably enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 439, 135685 (2022).
Dong, H. et al. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 79, 128–146 (2015).
Abutalib, M. et al. Fe3O4/Co3O4–TiO2 S-scheme photocatalyst for degradation of organic pollutants and H2 production under natural sunlight. J. Market. Res. 20, 1043–1056 (2022).
Wei, Z. et al. Photocatalytic hydrogen evolution with simultaneous antibiotic wastewater degradation via the visible-light-responsive bismuth spheres-g-C3N4 nanohybrid: Waste to energy insight. Chem. Eng. J. 358, 944–954 (2019).
Moradi, M., Sene, R. A., Rahmani, F. & Rezakazemi, M. Efficient photodegradation of paraquat herbicide over TiO2-WO3 heterojunction embedded in diatomite matrix and process optimization. Environ. Sci. Pollut. Res., 1–19 (2023).
Zhao, S. Z. et al. Constructing BiOBr/TiO2 heterostructure nanotubes for enhanced adsorption/photocatalytic performance. J. Water Process Eng. 54, 103972 (2023).
Bose, B. A. et al. BiOBr-rice husk carbon composite for antibiotic degradation. Mater. Sci. Semicond. Process. 177, 108366 (2024).
Imam, S. S., Adnan, R. & Mohd Kaus, N. H. Room-temperature in situ synthesis of BiOBr/Bi2O3 composites for the catalytic degradation of ciprofloxacin using indoor fluorescent light illumination. SN Appl. Sci. 1, 845 (2019).
Xiao, L., Yang, Z., Zhu, H. & Yan, G. Nanoflower-like BiOBr/TiO2 p-n heterojunction composites for enhanced photodegradation of formaldehyde and dyes. Inorg. Chem. Commun. 146, 110167 (2022).
Wang, J. et al. TiO2/BiOBr 2D–2D heterostructure via in-situ approach for enhanced visible-light photocatalytic N2 fixation. Appl. Surf. Sci. 567, 150623 (2021).
Ghorbani, M., Nazar, A. R. S., Frahadian, M. & Khosravi, M. Facile synthesis of Z-scheme ZnO-nanorod@ BiOBr-nanosheet heterojunction as efficient visible-light responsive photocatalyst: The effect of electrolyte and scavengers. J. Photochem. Photobiol., A 429, 113930 (2022).
Senasu, T., Chankhanittha, T., Hemavibool, K. & Nanan, S. Solvothermal synthesis of BiOBr photocatalyst with an assistant of PVP for visible-light-driven photocatalytic degradation of fluoroquinolone antibiotics. Catal. Today 384, 209–227 (2022).
Qi, Y. et al. Degradation of multiple xanthates using highly efficient visible light-responsive BiOBr-TiO2 composite photocatalysts. J. Ind. Eng. Chem. 132, 461–473 (2024).
Yu, X. et al. A ternary photocatalyst of all-solid-state Z-scheme TiO2–Au–BiOBr for efficiently degrading various dyes. J. Alloy. Compd. 839, 155597 (2020).
Eskandari, P. et al. Photocatalytic activity of visible-light-driven L-Proline-TiO2/BiOBr nanostructured materials for dyes degradation: The role of generated reactive species. J. Environ. Manag. 326, 116691 (2023).
Fang, W. L., Liang, W. & Li, C. H. Preparation of Au-OVs-BiOBr-P25 Z-scheme photocatalyst and its photocatalytic performance in overall water splitting. J. Fuel Chem. Technol. 50, 446–454 (2022).
Dlamini, M. C. et al. Photocatalytic abatement of phenol on amorphous TiO2-BiOBr-bentonite heterostructures under visible light irradiation. J. Ind. Eng. Chem. 111, 419–436 (2022).
Haounati, R. et al. Design of direct Z-scheme superb magnetic nanocomposite photocatalyst Fe3O4/Ag3PO4@ Sep for hazardous dye degradation. Sep. Purif. Technol. 277, 119399 (2021).
Hu, X., Li, C., Song, J., Zheng, S. & Sun, Z. Multidimensional assembly of oxygen vacancy-rich amorphous TiO2-BiOBr-sepiolite composite for rapid elimination of formaldehyde and oxytetracycline under visible light. J. Colloid Interface Sci. 574, 61–73 (2020).
Mehrabanpour, N., Nezamzadeh-Ejhieh, A., Ghattavi, S. & Ershadi, A. A magnetically separable clinoptilolite supported CdS-PbS photocatalyst: Characterization and photocatalytic activity toward cefotaxime. Appl. Surf. Sci. 614, 156252 (2023).
Zhou, P. et al. Synthesis of clinoptilolite-supported BiOCl/TiO2 heterojunction nanocomposites with highly-enhanced photocatalytic activity for the complete degradation of xanthates under visible light. Chem. Eng. J. 407, 126697 (2021).
Hosseinzadeh, G., Ghasemian, N. & Zinatloo-Ajabshir, S. TiO2/graphene nanocomposite supported on clinoptilolite nanoplate and its enhanced visible light photocatalytic activity. Inorg. Chem. Commun. 136, 109144 (2022).
Rahmani, F., Haghighi, M. & Amini, M. The beneficial utilization of natural zeolite in preparation of Cr/clinoptilolite nanocatalyst used in CO2-oxidative dehydrogenation of ethane to ethylene. J. Ind. Eng. Chem. 31, 142–155 (2015).
Davari, N., Farhadian, M., Nazar, A. R. S. & Homayoonfal, M. Degradation of diphenhydramine by the photocatalysts of ZnO/Fe2O3 and TiO2/Fe2O3 based on clinoptilolite: Structural and operational comparison. J. Environ. Chem. Eng. 5, 5707–5720 (2017).
Sene, R. A., Moradi, G. & Sharifnia, S. Sono-dispersion of TiO2 nanoparticles over clinoptilolite used in photocatalytic hydrogen production: Effect of ultrasound irradiation during conventional synthesis methods. Ultrason. Sonochem. 37, 490–501 (2017).
Sene, R. A., Sharifnia, S. & Moradi, G. On the impact evaluation of various chemical treatments of support on the photocatalytic properties and hydrogen evolution of sonochemically synthesized TiO2/Clinoptilolite. Int. J. Hydrogen Energy 43, 695–707 (2018).
Deng, J. et al. Preparation of Z-scheme Ag/AgBr/BiOBr composite photocatalyst for effective removal of organic pollutants. Chem. Phys. 548, 111228 (2021).
Wei, X. X., Cui, H., Guo, S., Zhao, L. & Li, W. Hybrid BiOBr–TiO2 nanocomposites with high visible light photocatalytic activity for water treatment. J. Hazard. Mater. 263, 650–658 (2013).
Prabhu, C. A., Silambarasan, D., Sarika, R. & Selvam, V. Synthesis and characterization of TiO2. Mater. Today Proc. 64, 1793–1797 (2022).
Park, M., Kwak, B. S., Jo, S. W. & Kang, M. Effective CH4 production from CO2 photoreduction using TiO2/x mol% Cu–TiO2 double-layered films. Energy Convers. Manag. 103, 431–438 (2015).
Li, S. et al. Hydrochar-mediated photocatalyst Fe3O4/BiOBr@ HC for highly efficient carbamazepine degradation under visible LED light irradiation. Chem. Eng. J. 433, 134492 (2022).
Wang, R. et al. Synthesis and characterization of successive Z-scheme CdS/Bi2MoO6/BiOBr heterojunction photocatalyst with efficient performance for antibiotic degradation. J. Alloy. Compd. 870, 159385 (2021).
Xu, S., Gao, X., Xu, W., Jin, P. & Kuang, Y. Efficient photocatalytic degradation of commercial pharmaceutical contaminants of carbamazepine using BiOBr nanosheets under visible-light irradiation. Mater. Sci. Semicond. Process. 137, 106207 (2022).
Chen, X. et al. Efficient photocatalytic simultaneous removal of organic and heavy metal complex pollutants under visible light by crystal-facet-mediated TiO2/Ti3C2-BiOBr nanocomposites. J. Water Process Eng. 54, 104032 (2023).
Wang, Y., Sunarso, J., Zhao, B., Ge, C. & Chen, G. One-dimensional BiOBr nanosheets/TiO2 nanofibers composite: Controllable synthesis and enhanced visible photocatalytic activity. Ceram. Int. 43, 15769–15776 (2017).
Han, L., Li, B., Wen, H., Guo, Y. & Lin, Z. Photocatalytic degradation of mixed pollutants in aqueous wastewater using mesoporous 2D/2D TiO2 (B)-BiOBr heterojunction. J. Mater. Sci. Technol. 70, 176–184 (2021).
Zhao, Y. et al. Fabrication of BiOBr nanosheets@ TiO2 nanobelts p–n junction photocatalysts for enhanced visible-light activity. Appl. Surf. Sci. 365, 209–217 (2016).
Rashid, J. et al. Butterfly cluster like lamellar BiOBr/TiO2 nanocomposite for enhanced sunlight photocatalytic mineralization of aqueous ciprofloxacin. Sci. Total Environ. 665, 668–677 (2019).
Zhang, B. et al. TiO2-X mesoporous nanospheres/BiOI nanosheets S-scheme heterostructure for high efficiency, stable and unbiased photocatalytic hydrogen production. Chem. Eng. J. 446, 137138 (2022).
Wu, C. et al. Mechanistic study of B-TiO2/BiVO4 S-scheme heterojunction photocatalyst for tetracycline hydrochloride removal and H2 production. Sep. Purif. Technol. 312, 123398 (2023).
Sadeghzadeh-Attar, A. Boosting the photocatalytic ability of hybrid BiVO4-TiO2 heterostructure nanocomposites for H2 production by reduced graphene oxide (rGO). J. Taiwan Inst. Chem. Eng. 111, 325–336 (2020).
Geng, L. et al. Active sites modification and superior carriers separation synergistically boosted hydrogen production of Bi/Bi2MoO6/ZnIn2S4 non-noble metal S-scheme photocatalyst. J. Colloid Interface Sci. 629, 723–732 (2023).
Lv, J. et al. Bi SPR-promoted Z-scheme Bi2MoO6/CdS-diethylenetriamine composite with effectively enhanced visible light photocatalytic hydrogen evolution activity and stability. ACS Sustain. Chem. Eng. 6, 696–706 (2018).
Cavdar, O. et al. Photocatalytic hydrogen evolution from glycerol-water mixture under visible light over zinc indium sulfide (ZnIn2S4) nanosheets grown on bismuth oxychloride (BiOCl) microplates. J. Colloid Interface Sci. 640, 578–587 (2023).
Chang, C. J. et al. Electron transfer dynamics and enhanced H2 production activity of hydrangea-like BiOBr/Bi2S3-based photocatalysts with Cu-complex as a redox mediator. Appl. Surf. Sci. 576, 151870 (2022).
Chang, C. J., Chen, Y. C. & Tsai, Z. T. Effect of calcination induced phase transition on the photocatalytic hydrogen production activity of BiOI and Bi5O7I based photocatalysts. Int. J. Hydrogen Energy 47, 40777–40786 (2022).
Wang, Y. Q., Yang, C. & Gan, L. H. Preparation of direct Z-scheme Bi2WO6/TiO2 heterojunction by one-step solvothermal method and enhancement mechanism of photocatalytic H2 production. Int. J. Hydrogen Energy 48, 19372–19384 (2023).
Bariki, R. et al. In-situ synthesis of structurally oriented hierarchical UiO-66 (–NH2)/CdIn2S4/CaIn2S4 heterostructure with dual S-scheme engineering for photocatalytic renewable H2 production and asulam degradation. Sep. Purif. Technol. 314, 123558 (2023).
Gao, C. et al. Construction of ZnIn2S4/Bi2MoO6 heterojunction enhancement photocatalytic hydrogen evolution performance under visible light. Int. J. Hydrogen Energy 52, 90–99 (2024).
Abd-Rabboh, H. S., Benaissa, M., Hamdy, M. S., Ahmed, M. & Glal, M. Synthesis of an efficient, and recyclable mesoporous BiVO4/TiO2 direct Z-scheme heterojunction by sonochemical route for photocatalytic hydrogen production and photodegradation of rhodamine B dye in the visible region. Opt. Mater. 114, 110761 (2021).
Qiang, Z. et al. Iodine doped Z-scheme Bi2O2CO3/Bi2WO6 photocatalysts: Facile synthesis, efficient visible light photocatalysis, and photocatalytic mechanism. Chem. Eng. J. 403, 126327 (2021).
Ali, S. et al. Fabrication of BiFeO3-g-C3N4-WO3 Z-scheme heterojunction as highly efficient visible-light photocatalyst for water reduction and 2, 4-dichlorophenol degradation: Insight mechanism. J. Hazard. Mater. 397, 122708 (2020).
Acknowledgements
The authors gratefully acknowledge University of Kurdistan for the financial support of the project (without grant number).
Author information
Authors and Affiliations
Contributions
Avin Zandi: Investigation, Conceptualization, Methodology, Formal analysis, Writing original draft. Rojiar Akbari Sene: Supervision, Project administration, Visualization, Conceptualization, Review & Editing. Farhad Rahmani: Supervision, Project administration, Visualization, Conceptualization, Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zandi, A., Sene, R.A. & Rahmani, F. Design and construction of clinoptilolite-decorated BiOBr-TiO2 heterojunction as efficient and affordable photocatalyst for herbicide contaminated wastewater splitting. Sci Rep 15, 42720 (2025). https://doi.org/10.1038/s41598-025-26882-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-26882-5