Abstract
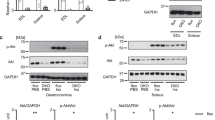
Age-related muscle mass is driven by a reduction in insulin sensitivity partly mediated by reduced amino acid and anabolic signalling kinetics. Insulin activates Akt-mTORC1 signalling in skeletal muscle, with inositol hexakisphosphate kinase 1 (IP6K1) shown to inhibit this signalling pathway in pre-diabetic humans. We aimed to compare muscle and plasma IP6K1 in young vs older adults and the possible role of IP6K1 in the anabolic response to protein and protein plus resistance exercise (RE). Nine young (24.9 ± 0.4 years) and nine older (66.2 ± 0.5 years), moderately active adults received primed continuous infusions of L-[ring-2H5]phenylalanine in basal and postprandial state. Blood and muscle biopsy samples were collected prior to and following ingestion of 25 g whey protein with or without knee extension exercise to examine skeletal muscle protein signalling and whole-body phenylalanine kinetics. Young adults had greater plasma IP6K1 at all time points. Older adults had reduced muscle IP6K1 at 120 min post-exercise. Muscle IP6K1 decreased 240 min postprandially in young adults compared with basal and there was no effect of exercise in either group. Older adults presented with reduced plasma and muscle IP6K1 in both postprandially and post-RE states, as well as reduced phenylalanine rate of disappearance for the same comparisons. IP6K1 may be involved in the reduction in amino acid metabolism, and the insulin-mediated response to protein and RE.
Similar content being viewed by others
Data availability
All datasets generated during and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- IP6K1:
-
Inositol hexakisphosphate kinase 1
- mTOR:
-
Mechanistic target of rapamycin
- mTORC1:
-
MTOR complex 1
- mTORC2:
-
MTOR complex 2
- Akt:
-
Protein kinase B
- PI3K:
-
Phosphoinositide 3-kinases
- IGF-1:
-
Insulin-like growth factor 1
- IGFR:
-
IGF receptor
- IRS-1:
-
Insulin receptor substrate 1
- Rheb, p70s6k :
-
Ribosomal protein S6 kinase beta-1
- 4E-BP1:
-
Eukaryotic translation initiation factor 4E-binding protein 1
- MPS:
-
Muscle protein synthesis
- MPB:
-
Muscle protein breakdown
- PA:
-
Phosphatidic acid
- PtdIns3P:
-
Phosphatidylinositol 3-phosphate
- LAT1:
-
L-type amino acid transporter 1
- NPB:
-
Net protein balance
- PDK1:
-
Phosphoinositide-dependent kinase 1
- IGFBP:
-
Insulin-like growth factor-binding protein
- PH:
-
Pleckstrin homology
- BMI:
-
Body mass index
- HOMAIR :
-
Homeostasis model assessment of insulin resistance
- RE:
-
Resistance exercise
- 1RM:
-
One-repetition maximum
References
Mackenzie, R. W. & Elliott, B. T. Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes, Metab. Synd. Obesity: Targets Ther. https://doi.org/10.2147/DMSO.S48260 (2014).
Naufahu, J. et al. High intensity exercise decreases IP6K1 muscle content & improves insulin sensitivity (SI2*) in glucose intolerant individuals. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/jc.2017-02019 (2019).
Chakraborty, A. et al. Inositol pyrophosphates inhibit akt signaling, thereby regulating insulin sensitivity and weight gain. Cell https://doi.org/10.1016/j.cell.2010.11.032 (2010).
Yu, W., Ye, C. & Greenberg, M. L. Inositol hexakisphosphate kinase 1 (IP6K1) regulates inositol synthesis in mammalian cells. J. Biol. Chem. https://doi.org/10.1074/jbc.M116.714816 (2016).
O’Neill, B. T. et al. Differential role of insulin/IGF-1 receptor signaling in muscle growth and glucose homeostasis. Cell Rep. https://doi.org/10.1016/j.celrep.2015.04.037 (2015).
Latres, E. et al. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/akt/mammalian target of rapamycin (PI3K/akt/mTOR) pathway. J. Biol. Chem. https://doi.org/10.1074/jbc.M407517200 (2005).
Barclay, R. D. et al. Ingestion of lean meat elevates muscle inositol hexakisphosphate kinase 1 protein content independent of a distinct post-prandial circulating proteome in young adults with obesity. Metabolism https://doi.org/10.1016/j.metabol.2019.153996 (2020).
Needham, E. J., Parker, B. L., Burykin, T., James, D. E. & Humphrey, S. J. Illuminating the dark phosphoproteome. Sci. Signal. 12(565), eaau8645. https://doi.org/10.1126/scisignal.aau8645 (2019).
Dickinson, J. M., Drummond, M. J., Coben, J. R., Volpi, E. & Rasmussen, B. B. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clin. Nutr. 32(2), 273–280 (2013).
Godin, G. & Shephard, R. J. A simple method to assess exercise behaviour in the community. Can. J. Appl. Sport Sci. 10(3), 141–146 (1985).
Jones, C. J., Rikli, R. E. & Beam, W. C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport https://doi.org/10.1080/02701367.1999.10608028 (1999).
Bohannon, R. W., Wang, Y. C. & Gershon, R. C. Two-minute walk test performance by adults 18 to 85 years: normative values, reliability, and responsiveness. Arch Phys. Med. Rehabil. https://doi.org/10.1016/j.apmr.2014.10.006 (2014).
Burd, N. A. et al. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. https://doi.org/10.1017/S0007114511006271 (2012).
Gray, N. et al. High-speed quantitative UPLC-MS analysis of multiple amines in human plasma and serum via precolumn derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate: application to acetaminophen-induced liver failure. Anal. Chem. 89(4), 2478–2487 (2017).
Romero-Calvo, I. et al. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. https://doi.org/10.1016/j.ab.2010.02.036 (2010).
Gastaldelli, A. C., Wolfe, A. R. & RR.,. Assessment of methods for improving tracer estimation of non-steady-state rate of appearance. J. Appl. Physiol. 87, 1813–1822 (1999).
Yang, M. et al. Inactivation of myostatin delays senescence TREX1-SASP bovine skeletal muscle cells. Int. J. Mol. Sci. 12(25), 5277. https://doi.org/10.3390/ijms25105277 (2024).
Volpi, E., Sheffield-Moore, M., Rasmussen, B. B. & Wolfe, R. R. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA https://doi.org/10.1001/jama.286.10.1206 (2001).
Padmanabhan, U., Dollins, D. E., Fridy, P. C., York, J. D. & Downes, C. P. Characterization of a selective inhibitor of inositol hexakisphosphate kinases. J. Biol. Chem. https://doi.org/10.1074/jbc.M900752200 (2009).
Parker, L., Trewin, A., Levinger, I., Shaw, C. S. & Stepto, N. K. The effect of exercise-intensity on skeletal muscle stress kinase and insulin protein signaling. PLoS ONE https://doi.org/10.1371/journal.pone.0171613 (2017).
Cuthbertson, D. et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J https://doi.org/10.1096/fj.04-2640fje (2005).
Drummond, M. J. et al. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol https://doi.org/10.1152/japplphysiol.01408.2010 (1985).
Müller, L., Fülop, T. & Pawelec, G. Immunosenescence in vertebrates and invertebrates. Immune Ageing https://doi.org/10.1186/1742-4933-10-12 (2013).
Beals, J. W. et al. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults, 2. Am. J. Clin. Nutr. https://doi.org/10.3945/ajcn.116.130385 (2016).
Kumar, V. et al. Muscle protein synthetic responses to exercise: effects of age, volume, and intensity. J. Gerontol. Series A: Biomed. Sci. Med. Sci. https://doi.org/10.1093/gerona/gls141 (2012).
Schulze, M. B. Metabolic health in normal-weight and obese individuals. Diabetologia 62, 558–566. https://doi.org/10.1007/s00125-018-4787-8 (2019).
Maruyama, Y., Ikeda, C., Wakabayashi, K., Ato, S. & Ogasawara, R. High-intensity muscle contraction-mediated increases in Akt1 and Akt2 phosphorylation do not contribute to mTORC1 activation and muscle protein synthesis. J. Appl. Physiol. https://doi.org/10.1152/japplphysiol.00578.2019 (1985).
Moore, D. R. et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. https://doi.org/10.3945/ajcn.2008.26401 (2008).
Moore, D. R. et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glu103 (2015).
Kumar, V. et al. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. https://doi.org/10.1113/jphysiol.2008.164483 (2008).
Koopman, R. et al. Dietary protein digestion and absorption rates and the subsequent postprandial muscle protein synthetic response do not differ between young and elderly men. J. Nutrition https://doi.org/10.3945/jn.109.109173 (2009).
Camera, D. M. et al. Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise. J. Appl. Physiol. 109(6), 1697–1706. https://doi.org/10.1152/japplphysiol.00582.2010 (2010).
Greenhaff, P. L. et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am. J. Physiol.-Endocrinol. Metab. 295(3), E595–E604. https://doi.org/10.1152/ajpendo.90411.2008 (2008).
Wagenmakers, A. J. M. Tracers to investigate protein and amino acid metabolism in human subjects. Proc. Nutr. Soc. 58(4), 987–1000. https://doi.org/10.1017/S0029665199001302 (1999).
Wolfe, R. R. Control of muscle protein breakdown: Effects of activity and nutritional states. Int. J. Sport Nutr. Exerc. Metab. 11(S1), S164–S169. https://doi.org/10.1123/ijsnem.11.s1.s164 (2001).
Gray, N. et al. High-speed quantitative UPLC-MS analysis of multiple amines in human plasma and serum via precolumn derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate: application to acetaminophen-induced liver failure. Anal. Chem. https://doi.org/10.1021/acs.analchem.6b04623 (2017).
Behrends, V., Tredwell, G. D. & Bundy, J. G. A software complement to AMDIS for processing GC-MS metabolomic data. Anal. Biochem. https://doi.org/10.1016/j.ab.2011.04.009 (2011).
Barclay, R. D., Burd, N. A., Tyler, C., Tillin, N. A. & Mackenzie, R. W. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front. Nutr. https://doi.org/10.3389/fnut.2019.00146 (2019).
Author information
Authors and Affiliations
Contributions
RDB, NAB, CJT, NAT and RWM contributed to the conception and the design of the experiment. RDB, DEM, OA, VB, NMH and RWM contributed to collection, analysis, and interpretation of data. RDB, CJT, NAT, VB, NAB, NMH and RWM contributed to drafting or revising intellectual content of the manuscript. RB and RWAM had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barclay, R.D., Motei, D.E., Ancu, O. et al. Inositol hexakisphosphate kinase 1 is implicated in the insulin response to protein ingestion in older adults. Sci Rep (2026). https://doi.org/10.1038/s41598-026-35711-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-35711-2