Abstract
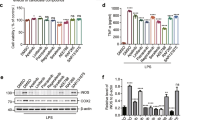
Neuroinflammatory pathways are emerging therapeutic targets for neurological conditions such as Parkinson’s disease (PD). Wnt-3a may exert anti-inflammatory effects via canonical pathway activation and β-catenin stabilization while dysregulation of the Wnt/β-catenin pathway has been implicated in the degeneration of dopamine neurons in PD. However, canonical pathway stimulation via application of Wnt-3a to protect against inflammation and dopaminergic degeneration has not been explored. We found Wnt-3a alone had no effect on pro-inflammatory TNF-α or IL-1β release from homeostatic primary microglia, however co-administration with LPS significantly increased TNF-α release beyond that seen with LPS alone. This exacerbation in TNF-α levels was not mediated by the NFκB pathway or activation of β-catenin. Canonical pathway inhibition via DKK1 showed no changes in TNF-α levels, however both SP600125 and U723122 were able to block Wnt-3a + LPS induced TNF-α release, implicating non-canonical pathways. Meanwhile, infusion of Wnt-3a in vivo did not alter dopaminergic or microglial populations in MPTP lesioned animals. Together, these findings suggest Wnt-3a may enhance pro-inflammatory TNF-α release via non-canonical signaling in inflammatory conditions, with minimal effect on homeostatic microglia. This demonstrates the importance of cellular context when identifying potential therapies for neurodegenerative diseases where neuroinflammation is a critical mediator of pathology.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Halleskog, C. et al. WNT signaling in activated microglia is Proinflammatory. Glia 59, 119–131. https://doi.org/10.1002/glia.21081 (2011).
Marchetti, B. & Pluchino, S. Wnt your brain be inflamed? Yes, it Wnt! Trends Mol. Med. 19, 144–156. https://doi.org/10.1016/j.molmed.2012.12.001 (2013).
Onyido, E. K., Sweeney, E. & Nateri, A. S. Wnt-signalling pathways and MicroRNAs network in carcinogenesis: experimental and bioinformatics approaches. Mol. Cancer. 15, 56. https://doi.org/10.1186/s12943-016-0541-3 (2016).
Clapham, D. E. Calcium signaling. Cell 131, 1047–1058. https://doi.org/10.1016/j.cell.2007.11.028 (2007).
Komiya, Y. & Habas, R. Wnt signal transduction pathways. Organogenesis 4, 68–75. https://doi.org/10.4161/org.4.2.5851 (2008).
Wei, L. et al. Activation of Wnt/beta-catenin pathway by exogenous Wnt1 protects SH-SY5Y cells against 6-hydroxydopamine toxicity. J. Mol. Neurosci. 49, 105–115. https://doi.org/10.1007/s12031-012-9900-8 (2013).
Castelo-Branco, G. et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc. Natl. Acad. Sci. U S A. 100, 12747–12752. https://doi.org/10.1073/pnas.1534900100 (2003).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452. https://doi.org/10.1038/nature01611 (2003).
L’Episcopo, F. et al. Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of parkinson’s disease. Neurobiol. Dis. 41, 508–527. https://doi.org/10.1016/j.nbd.2010.10.023 (2011).
Verheyen, E. M. & Gottardi, C. J. Regulation of Wnt/beta-catenin signaling by protein kinases. Dev. Dyn. 239, 34–44. https://doi.org/10.1002/dvdy.22019 (2010).
Dun, Y. et al. Inhibition of the canonical Wnt pathway by Dickkopf-1 contributes to the neurodegeneration in 6-OHDA-lesioned rats. Neurosci. Lett. 525, 83–88. https://doi.org/10.1016/j.neulet.2012.07.030 (2012).
L’Episcopo, F. et al. A Wnt1 regulated Frizzled-1/beta-Catenin signaling pathway as a candidate regulatory circuit controlling mesencephalic dopaminergic neuron-astrocyte crosstalk: therapeutical relevance for neuron survival and neuroprotection. Mol. Neurodegener. 6, 49. https://doi.org/10.1186/1750-1326-6-49 (2011).
Zhang, L. et al. Enhancing Beta-Catenin activity via GSK3beta Inhibition protects PC12 cells against rotenone toxicity through Nurr1 induction. PLoS One. 11, e0152931. https://doi.org/10.1371/journal.pone.0152931 (2016).
McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. Reactive microglia are positive for HLA-DR in the substantia Nigra of parkinson’s and alzheimer’s disease brains. Neurology 38, 1285–1291. https://doi.org/10.1212/wnl.38.8.1285 (1988).
Stefanova, N. Microglia in parkinson’s disease. J. Parkinsons Dis. 12, S105–S112. https://doi.org/10.3233/JPD-223237 (2022).
Lai, T. Microglial Inhibition alleviates alpha-synuclein propagation and neurodegeneration in parkinson’s disease mouse model. NPJ Parkinsons Dis. 10, 32. https://doi.org/10.1038/s41531-024-00640-2 (2024).
Liu, Q. et al. Single-cell sequencing of the substantia Nigra reveals microglial activation in a model of MPTP. Front. Aging Neurosci. 16, 1390310. https://doi.org/10.3389/fnagi.2024.1390310 (2024).
Lofrumento, D. D. et al. MPTP-induced neuroinflammation increases the expression of pro-inflammatory cytokines and their receptors in mouse brain. Neuroimmunomodulation 18, 79–88. https://doi.org/10.1159/000320027 (2011).
Stayte, S. et al. Activin A inhibits MPTP and LPS-Induced increases in inflammatory cell populations and loss of dopamine neurons in the mouse midbrain in vivo. PLoS One. 12, e0167211. https://doi.org/10.1371/journal.pone.0167211 (2017).
Collins, L. M., Toulouse, A., Connor, T. J. & Nolan, Y. M. Contributions of central and systemic inflammation to the pathophysiology of parkinson’s disease. Neuropharmacology 62, 2154–2168. https://doi.org/10.1016/j.neuropharm.2012.01.028 (2012).
Stephenson, J., Nutma, E., van der Valk, P. & Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 154, 204–219. https://doi.org/10.1111/imm.12922 (2018).
Yang, Y. & Zhang, Z. Microglia and Wnt pathways: prospects for inflammation in alzheimer’s disease. Front. Aging Neurosci. 12, 110. https://doi.org/10.3389/fnagi.2020.00110 (2020).
Gollamudi, S. et al. Concordant signaling pathways produced by pesticide exposure in mice correspond to pathways identified in human parkinson’s disease. PLoS One. 7, e36191. https://doi.org/10.1371/journal.pone.0036191 (2012).
Kwok, J. B. et al. GSK3B polymorphisms alter transcription and splicing in parkinson’s disease. Ann. Neurol. 58, 829–839. https://doi.org/10.1002/ana.20691 (2005).
L’Episcopo, F. et al. Wnt/beta-catenin signaling is required to rescue midbrain dopaminergic progenitors and promote neurorepair in ageing mouse model of parkinson’s disease. Stem Cells. 32, 2147–2163. https://doi.org/10.1002/stem.1708 (2014).
Ohnuki, T., Nakamura, A., Okuyama, S. & Nakamura, S. Gene expression profiling in progressively MPTP-lesioned macaques reveals molecular pathways associated with sporadic parkinson’s disease. Brain Res. 1346, 26–42. https://doi.org/10.1016/j.brainres.2010.05.066 (2010).
Zhang, L. et al. Targeted methylation sequencing reveals dysregulated Wnt signaling in Parkinson disease. J. Genet. Genomics. 43, 587–592. https://doi.org/10.1016/j.jgg.2016.05.002 (2016).
Manicassamy, S. et al. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 329, 849–853. https://doi.org/10.1126/science.1188510 (2010).
Neumann, J. et al. Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. FASEB J. 24, 4599–4612. https://doi.org/10.1096/fj.10-160994 (2010).
Sun, J. et al. Crosstalk between NF-kappaB and beta-catenin pathways in bacterial-colonized intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G129–137. https://doi.org/10.1152/ajpgi.00515.2004 (2005).
van Dijk, E. M. et al. Noncanonical WNT-5B signaling induces inflammatory responses in human lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 310, L1166–1176. https://doi.org/10.1152/ajplung.00226.2015 (2016).
Ma, B. & Hottiger, M. O. Crosstalk between Wnt/beta-Catenin and NF-kappaB signaling pathway during inflammation. Front. Immunol. 7, 378. https://doi.org/10.3389/fimmu.2016.00378 (2016).
Saadeddin, A., Babaei-Jadidi, R., Spencer-Dene, B. & Nateri, A. S. The links between transcription, beta-catenin/JNK signaling, and carcinogenesis. Mol. Cancer Res. 7, 1189–1196. https://doi.org/10.1158/1541-7786.MCR-09-0027 (2009).
Bikkavilli, R. K., Feigin, M. E. & Malbon, C. C. p38 mitogen-activated protein kinase regulates canonical Wnt-beta-catenin signaling by inactivation of GSK3beta. J. Cell. Sci. 121, 3598–3607. https://doi.org/10.1242/jcs.032854 (2008).
DiSabato, D. J., Quan, N. & Godbout, J. P. Neuroinflammation: the devil is in the details. J. Neurochem. 139 (Suppl 2), 136–153. https://doi.org/10.1111/jnc.13607 (2016).
Marchetti, B. et al. Parkinson’s disease, aging and adult neurogenesis: Wnt/beta-catenin signalling as the key to unlock the mystery of endogenous brain repair. Aging Cell. 19, e13101. https://doi.org/10.1111/acel.13101 (2020).
Tanaka, T. et al. General anesthetics inhibit LPS-induced IL-1beta expression in glial cells. PLoS One. 8, e82930. https://doi.org/10.1371/journal.pone.0082930 (2013).
Panicker, N. et al. Fyn kinase regulates microglial neuroinflammatory responses in cell culture and animal models of parkinson’s disease. J. Neurosci. 35, 10058–10077. https://doi.org/10.1523/JNEUROSCI.0302-15.2015 (2015).
Pocivavsek, A., Burns, M. P. & Rebeck, G. W. Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase. Glia 57, 444–453. https://doi.org/10.1002/glia.20772 (2009).
Zhu, L. et al. Anti-inflammatory activities of phospholipase C inhibitor U73122: Inhibition of monocyte-to-macrophage transformation and LPS-induced pro-inflammatory cytokine expression. Int. Immunopharmacol. 29, 622–627. https://doi.org/10.1016/j.intimp.2015.09.019 (2015).
Schaale, K., Neumann, J., Schneider, D., Ehlers, S. & Reiling, N. Wnt signaling in macrophages: augmenting and inhibiting mycobacteria-induced inflammatory responses. Eur. J. Cell. Biol. 90, 553–559 (2011).
Wetzel, A. et al. Dysregulated Wnt and NFAT signaling in a parkinson’s disease LRRK2 G2019S knock-in model. Sci. Rep. 14, 12393. https://doi.org/10.1038/s41598-024-63130-8 (2024).
Clark, I. A., Alleva, L. M. & Vissel, B. The roles of TNF in brain dysfunction and disease. Pharmacol. Ther. 128, 519–548. https://doi.org/10.1016/j.pharmthera.2010.08.007 (2010).
Clark, I. A. & Vissel, B. Therapeutic implications of how TNF links Apolipoprotein E, phosphorylated tau, alpha-synuclein, amyloid-beta and insulin resistance in neurodegenerative diseases. Br. J. Pharmacol. 175, 3859–3875. https://doi.org/10.1111/bph.14471 (2018).
Di Lazzaro, G. et al. Differential profiles of serum cytokines in parkinson’s disease according to disease duration. Neurobiol. Dis. 190, 106371. https://doi.org/10.1016/j.nbd.2023.106371 (2024).
Forlenza, O. V. et al. Increased serum IL-1beta level in alzheimer’s disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 28, 507–512. https://doi.org/10.1159/000255051 (2009).
Hannoush, R. N. Kinetics of Wnt-driven beta-catenin stabilization revealed by quantitative and Temporal imaging. PLoS One. 3, e3498. https://doi.org/10.1371/journal.pone.0003498 (2008).
Ito, M. et al. SP600125 inhibits cap-dependent translation independently of the c-Jun N-terminal kinase pathway. Cell. Struct. Funct. 36, 27–33. https://doi.org/10.1247/csf.10025 (2011).
Macmillan, D. & McCarron, J. G. The phospholipase C inhibitor U-73122 inhibits Ca(2+) release from the intracellular sarcoplasmic reticulum Ca(2+) store by inhibiting Ca(2+) pumps in smooth muscle. Br. J. Pharmacol. 160, 1295–1301. https://doi.org/10.1111/j.1476-5381.2010.00771.x (2010).
Schildge, S., Bohrer, C., Beck, K. & Schachtrup, C. Isolation and culture of mouse cortical astrocytes. J. Vis. Exp. https://doi.org/10.3791/50079 (2013).
Stayte, S. et al. The Kainate receptor antagonist UBP310 but not single deletion of GluK1, GluK2, or GluK3 subunits, inhibits MPTP-induced degeneration in the mouse midbrain. Exp. Neurol. 323, 113062. https://doi.org/10.1016/j.expneurol.2019.113062 (2020).
Gundersen, H. J. & Jensen, E. B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 147, 229–263. https://doi.org/10.1111/j.1365-2818.1987.tb02837.x (1987).
Paxinos, G. & Franklin, K. The Mouse Brain in Stereotaxic Coordinates 2 edn (Academic, 2001).
Funding
This study was supported by The Australian Government Research Training Program Scholarship to Gabrielle Federici; The Helen and David Baffsky Fellowship to Sandy Stayte; The Boyarsky family; Andrew Michael and Michele Brooks; John and Debbie Schaffer; Richard Gelski; Alex Sundich and Bridge Street Capital Partners; Doug Battersby and family; David King and family; Harry Holden; Tony and Vivian Howland-Rose; The ISG Foundation; Stanley and Charmaine Roth; Richard, Adrian and Tom O’Connor; Marnie and Gary Perlstein; David Schwartz and Stephen Young.
Author information
Authors and Affiliations
Contributions
GF, SS, PR, and BV conceptualized the studies. GF performed all in vitro studies. GF and SS performed all in vivo studies. SS and BV provided supervision. All authors contributed to the writing of the manuscript and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Federici, G., Stayte, S., Rentsch, P. et al. Wnt-3a exacerbates production of TNF-α in LPS stimulated microglia independent of the β-catenin canonical pathway. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37653-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37653-1