Abstract
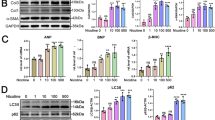
Chronic exposure to nicotine significantly exacerbates periodontitis, a prevalent inflammatory disease, by inducing cellular processes such as autophagy and inflammation in gingival fibroblasts. Current therapies often fail to fully address these cellular alterations in smokers, highlighting a need for innovative therapeutic and regenerative approaches. This study explores the therapeutic potential of Platelet-Rich Plasma (PRP), a blood-derived product, to modulate nicotine-induced biological activities in primary gingival fibroblasts, particularly in the case of periodontitis in smokers. Gingival fibroblasts were treated with increasing concentrations of nicotine, which led to senescence and autophagy. Nicotine at high concentrations triggered cellular vacuolization, and a decrease in metabolism, viability and proliferation. Concomitant cell treatment with 10% PRP reversed nicotine effects and significantly increased cell migration potential. In Caenorhabditis elegans, PRP reduced the nicotine-induced autophagic activity. A screening of the gingival fibroblast secretome revealed a modulation of autophagy-related cytokines in response to nicotine and/or PRP. The findings demonstrate that PRP could effectively inhibit nicotine-induced autophagy in gingival fibroblasts, offering insights into its possible use as a therapeutic tool for managing periodontitis in smokers. The study underscores the potential of PRP in altering disease progression by modulating key cellular processes affected by smoking.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article. Experiments were realized in accordance with ARRIVE guidelines.
References
Kinane, D. F., Stathopoulou, P. G. & Papapanou, P. N. Periodontal diseases. Nat. Rev. Dis. Prim. 3, 17038. https://doi.org/10.1038/nrdp.2017.38 (2017).
Kinane, D. F. Causation and pathogenesis of periodontal disease. Periodontology 2000(25), 8–20. https://doi.org/10.1034/j.1600-0757.2001.22250102.x (2001).
Giannopoulou, C., Geinoz, A. & Cimasoni, G. Effects of nicotine on periodontal ligament fibroblasts in vitro. J. Clin. Periodontol. 26, 49–55. https://doi.org/10.1034/j.1600-051x.1999.260109.x (1999).
Kang, S. W. et al. Effects of nicotine on apoptosis in human gingival fibroblasts. Arch. Oral Biol. 56, 1091–1097. https://doi.org/10.1016/j.archoralbio.2011.03.016 (2011).
Pabst, M. J. et al. Inhibition of neutrophil and monocyte defensive functions by nicotine. J. Periodontol. 66, 1047–1055. https://doi.org/10.1902/jop.1995.66.12.1047 (1995).
Holliday, R. S., Campbell, J. & Preshaw, P. M. Effect of nicotine on human gingival, periodontal ligament and oral epithelial cells. a systematic review of the literature. J. Dent. 86(81), 88. https://doi.org/10.1016/j.jdent.2019.05.030 (2019).
Apatzidou, D. A. The role of cigarette smoking in periodontal disease and treatment outcomes of dental implant therapy. Periodontology 2000(90), 45–61. https://doi.org/10.1111/prd.12449 (2022).
Gomez-Virgilio, L. et al. Autophagy: a key regulator of homeostasis and disease: an overview of molecular mechanisms and modulators. Cells https://doi.org/10.3390/cells11152262 (2022).
Zhang, W. et al. Platelet-Rich plasma for the treatment of tissue infection: preparation and clinical evaluation. Tissue Eng. Part B, Rev. 25(225), 236. https://doi.org/10.1089/ten.TEB.2018.0309 (2019).
Arpornmaeklong, P., Kochel, M., Depprich, R., Kubler, N. R. & Wurzler, K. K. Influence of platelet-rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells An in vitro study. Int. J. Oral Maxillofac. Surg. 33(60), 70. https://doi.org/10.1054/ijom.2003.0492 (2004).
Berndt, S., Turzi, A., Pittet-Cuenod, B. & Modarressi, A. Autologous Platelet-Rich Plasma (CuteCell PRP) safely boosts in vitro human fibroblast expansion. Tissue Eng. Part A 25, 1550–1563. https://doi.org/10.1089/ten.TEA.2018.0335 (2019).
Kobayashi, E. et al. Effects of platelet rich plasma (PRP) on human gingival fibroblast, osteoblast and periodontal ligament cell behaviour. BMC Oral Health 17, 91. https://doi.org/10.1186/s12903-017-0381-6 (2017).
Wang, X., Zhang, Y., Choukroun, J., Ghanaati, S. & Miron, R. J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 29, 48–55. https://doi.org/10.1080/09537104.2017.1293807 (2018).
Xu, J., Gou, L., Zhang, P., Li, H. & Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 65, 131–142. https://doi.org/10.1111/adj.12754 (2020).
Sun, J. et al. Emerging roles of platelet concentrates and platelet-derived extracellular vesicles in regenerative periodontology and implant dentistry. APL Bioeng. 6, 031503. https://doi.org/10.1063/5.0099872 (2022).
Heasman, L. et al. The effect of smoking on periodontal treatment response: a review of clinical evidence. J. Clin. Periodontol. 33, 241–253. https://doi.org/10.1111/j.1600-051X.2006.00902.x (2006).
Kinane, D. F. & Chestnutt, I. G. Smoking and periodontal disease. Crit. Rev. Oral Biol. Med. 11, 356–365. https://doi.org/10.1177/10454411000110030501 (2000).
Raulin, L. A., McPherson, J. C. 3rd., McQuade, M. J. & Hanson, B. S. The effect of nicotine on the attachment of human fibroblasts to glass and human root surfaces in vitro. J. Periodontol. 59, 318–325. https://doi.org/10.1902/jop.1988.59.5.318 (1988).
Tipton, D. A. & Dabbous, M. K. Effects of nicotine on proliferation and extracellular matrix production of human gingival fibroblasts in vitro. J. Periodontol. 66, 1056–1064. https://doi.org/10.1902/jop.1995.66.12.1056 (1995).
Colombo, G. et al. Oxidative damage in human gingival fibroblasts exposed to cigarette smoke. Free Radic. Biol. Med. 52, 1584–1596. https://doi.org/10.1016/j.freeradbiomed.2012.02.030 (2012).
Kim, B. S. et al. Effects of nicotine on proliferation and osteoblast differentiation in human alveolar bone marrow-derived mesenchymal stem cells. Life Sci. 90, 109–115. https://doi.org/10.1016/j.lfs.2011.10.019 (2012).
Chioran, D. et al. Nicotine exerts cytotoxic effects in a panel of healthy cell lines and strong irritating potential on blood vessels. Int. J. Environ. Res. Public Health https://doi.org/10.3390/ijerph19148881 (2022).
Benowitz, N. L., Bernert, J. T., Caraballo, R. S., Holiday, D. B. & Wang, J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am. J. Epidemiol. 169, 236–248. https://doi.org/10.1093/aje/kwn301 (2009).
Benowitz, N. L., Hukkanen, J. & Jacob, P. 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. https://doi.org/10.1007/978-3-540-69248-5_2 (2009).
Marinucci, L., Bodo, M., Balloni, S., Locci, P. & Baroni, T. Sub-toxic nicotine concentrations affect extracellular matrix and growth factor signaling gene expressions in human osteoblasts. J. Cell. Physiol. 229, 2038–2048. https://doi.org/10.1002/jcp.24661 (2014).
Caliri, A. W., Tommasi, S. & Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 787, 108365. https://doi.org/10.1016/j.mrrev.2021.108365 (2021).
Xing, R. et al. Low-dose nicotine promotes autophagy of cardiomyocytes by upregulating HO-1 expression. Biochem. Biophys. Res. Commun. 522, 1015–1021. https://doi.org/10.1016/j.bbrc.2019.11.086 (2020).
Wu, X. et al. Dual regulation of nicotine on NLRP3 inflammasome in macrophages with the involvement of lysosomal destabilization, ROS and alpha7nAChR. Inflammation 48, 61–74. https://doi.org/10.1007/s10753-024-02036-z (2025).
Rinaldi, S. et al. Oral nicotine pouches with an aftertaste? Part 2: in vitro toxicity in human gingival fibroblasts. Arch. Toxicol. 97, 2343–2356. https://doi.org/10.1007/s00204-023-03554-9 (2023).
Bielecki, T. & Dohan Ehrenfest, D. M. Platelet-rich plasma (PRP) and Platelet-Rich Fibrin (PRF): surgical adjuvants, preparations for in situ regenerative medicine and tools for tissue engineering. Curr. Pharm. Biotechnol. 13, 1121–1130. https://doi.org/10.2174/138920112800624292 (2012).
Rebimbas Guerreiro, S. et al. Platelet-Rich plasma and platelet-rich fibrin in endodontics: a scoping review. Int. J. Mol. Sci. https://doi.org/10.3390/ijms26125479 (2025).
Cho, E. B. et al. Effect of platelet-rich plasma on proliferation and migration in human dermal fibroblasts. J. Cosmet. Dermatol. 18, 1105–1112. https://doi.org/10.1111/jocd.12780 (2019).
Taylor, L. M. & Khachigian, L. M. Induction of platelet-derived growth factor B-chain expression by transforming growth factor-beta involves transactivation by Smads. J. Biol. Chem. 275, 16709–16716. https://doi.org/10.1074/jbc.275.22.16709 (2000).
Giarratana, A. O., Prendergast, C. M., Salvatore, M. M. & Capaccione, K. M. TGF-beta signaling: critical nexus of fibrogenesis and cancer. J. Transl. Med. 22, 594. https://doi.org/10.1186/s12967-024-05411-4 (2024).
Mehrabani, D., Seghatchian, J. & Acker, J. P. Platelet rich plasma in treatment of musculoskeletal pathologies. Transfus Apher. Sci. 58, 102675. https://doi.org/10.1016/j.transci.2019.102675 (2019).
Li, C. X. et al. Inhibiting autophagy promotes collagen degradation by regulating matrix metalloproteinases in pancreatic stellate cells. Life Sci. 208, 276–283. https://doi.org/10.1016/j.lfs.2018.07.049 (2018).
Gao, Y. et al. ATG5-regulated CCL2/MCP-1 production in myeloid cells selectively modulates anti-malarial CD4(+) Th1 responses. Autophagy 20, 1398–1417. https://doi.org/10.1080/15548627.2024.2319512 (2024).
Kolattukudy, P. E. & Niu, J. Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ. Res. 110, 174–189. https://doi.org/10.1161/CIRCRESAHA.111.243212 (2012).
Korhonen, E. et al. SQSTM1/p62 regulates the production of IL-8 and MCP-1 in IL-1beta-stimulated human retinal pigment epithelial cells. Cytokine 116, 70–77. https://doi.org/10.1016/j.cyto.2018.12.015 (2019).
An, Q., Yan, W., Zhao, Y. & Yu, K. Enhanced neutrophil autophagy and increased concentrations of IL-6, IL-8, IL-10 and MCP-1 in rheumatoid arthritis. Int Immunopharmacol 65, 119–128. https://doi.org/10.1016/j.intimp.2018.09.011 (2018).
Qin, B., Zhou, Z., He, J., Yan, C. & Ding, S. IL-6 inhibits starvation-induced autophagy via the STAT3/Bcl-2 signaling pathway. Sci. Rep. 5, 15701. https://doi.org/10.1038/srep15701 (2015).
Festa, B. P. et al. Microglial-to-neuronal CCR5 signaling regulates autophagy in neurodegeneration. Neuron 111, 2021–2037. https://doi.org/10.1016/j.neuron.2023.04.006 (2023).
Zeng, Z., Lan, T., Wei, Y. & Wei, X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. 9, 12–27. https://doi.org/10.1016/j.gendis.2021.08.004 (2022).
Veriepe-Salerno, J. et al. 2023 MALT-1 shortens lifespan by inhibiting autophagy in the intestine of C. elegans. Autophagy Rep. 2, 2277584. https://doi.org/10.1080/27694127.2023.2277584 (2023).
Fang, Y. & Svoboda, K. K. Nicotine inhibits myofibroblast differentiation in human gingival fibroblasts. J. Cell Biochem. 95, 1108–1119. https://doi.org/10.1002/jcb.20473 (2005).
Malhotra, R., Kapoor, A., Grover, V. & Kaushal, S. Nicotine and periodontal tissues. J. Indian Soc. Periodontol. 14, 72–79. https://doi.org/10.4103/0972-124X.65442 (2010).
Giannelli, A. et al. Utilization of platelet-rich plasma in oral surgery: a systematic review of the literature. J. Clin. Med. https://doi.org/10.3390/jcm14082844 (2025).
Gomri, F., Vischer, S., Turzi, A. & Berndt, S. Swiss medical devices for autologous regenerative medicine: from innovation to clinical validation. Pharmaceutics https://doi.org/10.3390/pharmaceutics14081617 (2022).
Omondi, R. O., Bellam, R., Ojwach, S. O., Jaganyi, D. & Fatokun, A. A. Palladium(II) complexes of tridentate bis(benzazole) ligands: structural, substitution kinetics, DNA interactions and cytotoxicity studies. J. Inorg. Biochem. 210, 111156. https://doi.org/10.1016/j.jinorgbio.2020.111156 (2020).
Thome, M. P. et al. Ratiometric analysis of acridine orange staining in the study of acidic organelles and autophagy. J. Cell Sci. 129, 4622–4632. https://doi.org/10.1242/jcs.195057 (2016).
Traganos, F. & Darzynkiewicz, Z. Lysosomal proton pump activity: supravital cell staining with acridine orange differentiates leukocyte subpopulations. Methods Cell Biol. 41, 185–194. https://doi.org/10.1016/s0091-679x(08)61717-3 (1994).
T., S. in Wormbook 1-11 (2006).
Berndt, S. et al. Angiogenesis Is differentially modulated by platelet-derived products. Biomedicines https://doi.org/10.3390/biomedicines9030251 (2021).
Acknowledgements
The C. elegans strains were kindly provided by the C. elegans Genetics Center (University of Minnesota, MN, USA). A great thanks to Nicolas Liaudet, who coded the script for picture quantification on QuPath and to the Bioimaging facility of the UNIGE for their help. We thank Vanessa Fétaud Lapierre and Gaël Vieille for allowing us the use of the UFA facility and its equipment.
Author information
Authors and Affiliations
Contributions
S.B. and C.G. designed the study. S.B, JA.C, performed the cell experiments. J.VS. designed and performed the experiments in *C. elegans* . A.T, M.C, S.V and C.G. contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare the following competing interest: S.B. is head project manager, cell therapy, at Regen Lab; an employee of ouis employed by and A.T. is the CEO of Regen Lab SA. The authors receive salary from Regen Lab SA. The study received financial support, materials and reagents from Regen Lab SA. Regen Lab SA develops and commercializes products in the field related to this study and could reasonably benefit from its findings. All other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vérièpe-Salerno, J., Cancela, J.A., Vischer, S. et al. Platelet-rich plasma promotes cellular recovery from nicotine-induced toxicity via autophagy modulation. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38188-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38188-1