Abstract
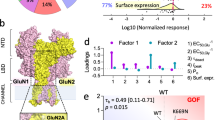
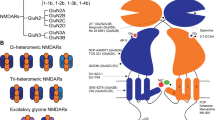
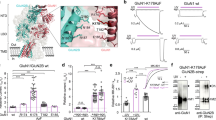
Gene expression data indicate that during human brain development, neurons change the NMDA receptor (NMDAR) subunit composition to modulate their function, favouring the GluN2A subunit over GluN2B—a hallmark of neuronal maturation. However, evidence supporting this phenomenon in human iPSC-derived neurons remains elusive. Here, using two differentiation methods in parallel (BrainPhys Neuronal Medium, BPM, and Neural Maintenance Medium, NMM), we provide evidence of increased synaptic localization of NMDARs during neuronal maturation and that GluN2A subunit is crucial for the NMDA physiological function-inducing inward currents and calcium entrance at 60 days of differentiation. Calcium responses to specific agonists, particularly NMDA, were elevated in cells cultured under BPM conditions. This is likely attributable to their more mature neuronal phenotype and the RNA-seq identified upregulation of genes involved in intracellular calcium signaling proteins. Our results offer insight into how glutamate receptor subunits mature during brain development, delineating approaches to study NMDAR activity in health and disease.
Similar content being viewed by others
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Greig, L. C., Woodworth, M. B., Galazo, M. J., Padmanabhan, H. & Macklis, J. D. Molecular logic of neocortical projection neuron specification, development and diversity. Nat. Rev. Neurosci. 14, 755–769 (2013).
Prince, G. S., Reynolds, M., Martina, V. & Sun, H. Gene-environmental regulation of the postnatal post-mitotic neuronal maturation. Trends Genet. 40, 480–494 (2024).
Wallace, J. L. & Pollen, A. A. Human neuronal maturation comes of age: Cellular mechanisms and species differences. Nat. Rev. Neurosci. 25, 7–29 (2024).
Hunt, D. L. & Castillo, P. E. Synaptic plasticity of NMDA receptors: Mechanisms and functional implications. Curr. Opin. Neurobiol. 22, 496–508 (2012).
Lee, L.-J., Lo, F.-S. & Erzurumlu, R. S. NMDA receptor-dependent regulation of axonal and dendritic branching. J. Neurosci. 25, 2304–2311 (2005).
Ikonomidou, C. et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1979(283), 70–74 (1999).
Sanz-Clemente, A., Nicoll, R. A. & Roche, K. W. Diversity in NMDA receptor composition. Neuroscientist 19, 62–75 (2013).
van der Aart, J. et al. Evaluation of the novel PET Tracer [11C]HACH242 for imaging the GluN2B NMDA receptor in non-human primates. Mol. Imaging Biol. 21, 676–685 (2019).
Paoletti, P., Bellone, C. & Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400 (2013).
Sanz-Clemente, A., Nicoll, R. A. & Roche, K. W. Diversity in NMDA receptor composition: Many regulators, many consequences. Neuroscientist 19, 62–75 (2013).
Scherzer, C. R. et al. Expression of N-Methyl-D-Aspartate receptor subunit mRNAs in the human brain: Hippocampus and cortex. J. Comp. Neurol. 390, 75–90 (1998).
Kornhuber, J., Mack-Burkhardt, F. & Riederer, P. Regional distribution of [3H]MK-801 binding sites in the human brain. Brain Res. 489, 397–399 (1989).
Pegasiou, C. M. et al. Age-dependent changes in synaptic NMDA receptor composition in adult human cortical neurons. Cereb. Cortex 30, 4246–4256 (2020).
Bagasrawala, I., Memi, F., Radonjić, V. & Zecevic, N. N. N -Methyl d-aspartate receptor expression patterns in the human fetal cerebral cortex. Cereb. Cortex https://doi.org/10.1093/cercor/bhw289 (2016).
Bar-Shira, O., Maor, R. & Chechik, G. Gene expression switching of receptor subunits in human brain development. PLoS Comput. Biol. 11, 20 (2015).
Jantzie, L. L. et al. Developmental expression of N-Methyl-d-aspartate (NMDA) receptor subunits in human white and gray matter: Potential mechanism of increased vulnerability in the immature brain. Cereb. Cortex 25, 482–495 (2015).
Dumas, T. C. Developmental regulation of cognitive abilities: Modified composition of a molecular switch turns on associative learning. Prog. Neurobiol. 76, 189–211 (2005).
Harrison, P. J. & Bannerman, D. M. GRIN2A (NR2A): A gene contributing to glutamatergic involvement in schizophrenia. Mol. Psychiatry 28, 3568–3572 (2023).
Mangano, G. D. et al. De novo GRIN2A variants associated with epilepsy and autism and literature review. Epilepsy Behav. 129, 108604 (2022).
Reutlinger, C. et al. Deletions in 16p13 including GRIN2A in patients with intellectual disability, various dysmorphic features, and seizure disorders of the rolandic region. Epilepsia 51, 1870–1873 (2010).
Maltese, M. et al. Early structural and functional plasticity alterations in a susceptibility period of DYT1 dystonia mouse striatum. Elife 7, e33331 (2018).
Sinclair, D. et al. Effects of sex and DTNBP1 (dysbindin) null gene mutation on the developmental GluN2B-GluN2A switch in the mouse cortex and hippocampus. J. Neurodev. Disord. 8, 14 (2016).
Barria, A. & Malinow, R. Subunit-specific NMDA receptor trafficking to synapses. Neuron 35, 345–353 (2002).
Flint, A. C., Maisch, U. S., Weishaupt, J. H., Kriegstein, A. R. & Monyer, H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J. Neurosci. 17, 2469–2476 (1997).
Sheng, M., Cummings, J., Roldan, L. A., Jan, Y. N. & Jan, L. Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368, 144–147 (1994).
Philpot, B. D. et al. Effect of transgenic overexpression of NR2B on NMDA receptor function and synaptic plasticity in visual cortex. Neuropharmacology 41, 762–770 (2001).
Yashiro, K. & Philpot, B. D. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 55, 1081–1094 (2008).
Baez, M. V., Cercato, M. C. & Jerusalinsky, D. A. NMDA receptor subunits change after synaptic plasticity induction and learning and memory acquisition. Neural Plast. 2018, 5093048 (2018).
Bellone, C. & Nicoll, R. A. Rapid bidirectional switching of synaptic NMDA receptors. Neuron 55, 779–785 (2007).
Franchini, L. et al. Linking NMDA receptor synaptic retention to synaptic plasticity and cognition. iScience 19, 927–939 (2019).
Thomas, C. G., Miller, A. J. & Westbrook, G. L. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J. Neurophysiol. 95, 1727–1734 (2006).
Kirson, E. D. & Yaari, Y. Synaptic NMDA receptors in developing mouse hippocampal neurones: Functional properties and sensitivity to ifenprodil. J. Physiol. 497, 437–455 (1996).
Law, A. J. et al. Expression of NMDA receptor NR1, NR2A and NR2B subunit mRNAs during development of the human hippocampal formation. Eur. J. Neurosci. 18, 1197–1205 (2003).
Tovar, K. R. & Westbrook, G. L. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J. Neurosci. 19, 4180–4188 (1999).
Stocca, G. & Vicini, S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J. Physiol. 507, 13–24 (1998).
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Satir, T. M. et al. Accelerated neuronal and synaptic maturation by BrainPhys medium increases Aβ secretion and alters Aβ peptide ratios from iPSC-derived cortical neurons. Sci. Rep. 10, 601 (2020).
Shi, Y., Kirwan, P. & Livesey, F. J. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat. Protoc. 7, 1836–1846 (2012).
Yen, Y.-P. et al. Dlk1-Dio3 locus-derived lncRNAs perpetuate postmitotic motor neuron cell fate and subtype identity. Elife 7, e38080 (2018).
Surmacz, B. et al. DLK1 promotes neurogenesis of human and mouse pluripotent stem cell-derived neural progenitors via modulating notch and BMP signalling. Stem Cell Rev. Rep. 8, 459–471 (2012).
Jensen, G. S., Leon-Palmer, N. E. & Townsend, K. L. Bone morphogenetic proteins (BMPs) in the central regulation of energy balance and adult neural plasticity. Metabolism 123, 154837 (2021).
Ioannides, Y., Lokulo-Sodipe, K., Mackay, D. J. G., Davies, J. H. & Temple, I. K. Temple syndrome: Improving the recognition of an underdiagnosed chromosome 14 imprinting disorder: an analysis of 51 published cases. J. Med. Genet. 51, 495–501 (2014).
Bakrania, P. et al. Mutations in BMP4 Cause Eye, Brain, and digit developmental anomalies: Overlap between the BMP4 and hedgehog signaling pathways. Am. J. Human Genet. 82, 304–319 (2008).
Roussel-Gervais, A. et al. Genetic knockout of NTRK2 by CRISPR/Cas9 decreases neurogenesis and favors glial progenitors during differentiation of neural progenitor stem cells. Front. Cell. Neurosci. 17, 1289966 (2023).
Gomez-Skarmeta, J. L. Xiro, a Xenopus homolog of the Drosophila Iroquois complex genes, controls development at the neural plate. EMBO J. 17, 181–190 (1998).
de la Calle-Mustienes, E., Glavic, A., Modolell, J. & Gómez-Skarmeta, J. L. Xiro homeoproteins coordinate cell cycle exit and primary neuron formation by upregulating neuronal-fate repressors and downregulating the cell-cycle inhibitor XGadd45-γ. Mech. Dev. 119, 69–80 (2002).
Cheng, C. W. et al. Zebrafish homologue irx1a is required for the differentiation of serotonergic neurons. Dev. Dyn. 236, 2661–2667 (2007).
Dou, Z., Son, J. E. & Hui, C. Irx3 and Irx5 - novel regulatory factors of postnatal hypothalamic neurogenesis. Front. Neurosci. 15, 763856 (2021).
Dong, D. et al. Stable expression of FoxA1 promotes pluripotent P19 embryonal carcinoma cells to be neural Stem-Like Cells. Gene Expr. 15, 153–162 (2012).
Karalay, Ö. et al. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. 108, 5807–5812 (2011).
Lavado, A., Lagutin, O. V., Chow, L. M. L., Baker, S. J. & Oliver, G. Prox1 Is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 8, e1000460 (2010).
Salman, M. M. et al. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 145, 64–75 (2022).
Hung, C.-C. et al. Astrocytic GAP43 induced by the TLR4/NF-κB/STAT3 axis attenuates astrogliosis-mediated microglial activation and neurotoxicity. J. Neurosci. 36, 2027–2043 (2016).
Liu, X., Bae, C., Gelman, B. B., Chung, J. M. & Tang, S.-J. A neuron-to-astrocyte Wnt5a signal governs astrogliosis during HIV-associated pain pathogenesis. Brain 145, 4108–4123 (2022).
Jiang, X., Knox, R., Pathipati, P. & Ferriero, D. Developmental localization of NMDA receptors, Src and MAP kinases in mouse brain. Neurosci. Lett. 503, 215–219 (2011).
Zhang, W., Ross, P. J., Ellis, J. & Salter, M. W. Targeting NMDA receptors in neuropsychiatric disorders by drug screening on human neurons derived from pluripotent stem cells. Transl. Psychiatry 12, 243 (2022).
Petralia, R. S. et al. Organization of NMDA receptors at extrasynaptic locations. Neuroscience 167, 68–87 (2010).
Gladding, C. M. & Raymond, L. A. Mechanisms underlying NMDA receptor synaptic/extrasynaptic distribution and function. Mol. Cell. Neurosci. 48, 308–320 (2011).
Groc, L. et al. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc. Natl. Acad. Sci. 103, 18769–18774 (2006).
Ivanov, A. et al. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J. Physiol. 572, 789–798 (2006).
Tovar, K. R. & Westbrook, G. L. Mobile NMDA receptors at hippocampal synapses. Neuron 34, 255–264 (2002).
Rosenmund, C., Feltz, A. & Westbrook, G. Synaptic NMDA receptor channels have a low open probability. J. Neurosci. 15, 2788–2795 (1995).
Watkins, J. C. Pharmacology of excitatory amino acid transmitters. Adv. Biochem. Psychopharmacol. 29, 205–212 (1981).
Fischer, G. et al. Ro 25–6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J. Pharmacol. Exp. Ther. 283, 1285–1292 (1997).
Auberson, Y. P. et al. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg. Med. Chem. Lett. 12, 1099–1102 (2002).
Brini, M., Calì, T., Ottolini, D. & Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 71, 2787–2814 (2014).
Grienberger, C. & Konnerth, A. Imaging calcium in neurons. Neuron 73, 862–885 (2012).
Zhu, S. et al. Mechanism of NMDA receptor inhibition and activation. Cell 165, 704–714 (2016).
Lee, H. C., Deng, Q. W. & Zhao, Y. J. The calcium signaling enzyme CD38 - a paradigm for membrane topology defining distinct protein functions. Cell Calcium 101, 102514 (2022).
Calcraft, P. J. et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600 (2009).
Sproul, A. A. et al. Characterization and molecular profiling of PSEN1 familial Alzheimer’s disease iPSC-derived neural progenitors. PLoS ONE 9, e84547 (2014).
Kondo, T. et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell 12, 487–496 (2013).
Israel, M. A. et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482, 216–220 (2012).
Hedegaard, A. et al. Pro-maturational effects of human iPSC-derived cortical astrocytes upon iPSC-derived cortical neurons. Stem Cell Rep. 15, 38–51 (2020).
Klapper, S. D. et al. Astrocyte lineage cells are essential for functional neuronal differentiation and synapse maturation in human iPSC-derived neural networks. Glia 67, 1893–1909 (2019).
Jackson, T. C., Kotermanski, S. E., Jackson, E. K. & Kochanek, P. M. BrainPhys® increases neurofilament levels in CNS cultures, and facilitates investigation of axonal damage after a mechanical stretch-injury in vitro. Exp. Neurol. 300, 232–246 (2018).
Bardy, C. et al. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc. Natl. Acad. Sci. 112, E2725 (2015).
von Bartheld, C. S., Bahney, J. & Herculano-Houzel, S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J. Comp. Neurol. 524, 3865–3895 (2016).
Shi, F., Corrales, C. E., Liberman, M. C. & Edge, A. S. B. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur. J. Neurosci. 26, 3016–3023 (2007).
Moon, B.-S. et al. Bone morphogenetic protein 4 stimulates neuronal differentiation of neuronal stem cells through the ERK pathway. Exp. Mol. Med. 41, 116 (2009).
Voss, L., Bartos, M., Elgueta, C. & Sauer, J. F. Interneuron function and cognitive behavior are preserved upon postnatal removal of Lhx6. Sci. Rep. 12, 4923 (2022).
Kim, D. W. et al. Gene regulatory networks controlling differentiation, survival, and diversification of hypothalamic Lhx6-expressing GABAergic neurons. Commun. Biol. 4, 95 (2021).
Batista-Brito, R., MacHold, R., Klein, C. & Fishell, G. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb. Cortex 18, 2306–2317 (2008).
Pai, E. L. L. et al. Mafb and c-Maf Have prenatal compensatory and postnatal antagonistic roles in cortical interneuron fate and function. Cell Rep. 26, 1157-1173.e5 (2019).
Matta, J. A. et al. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat. Neurosci. 16, 1032–1041 (2013).
Ruden, J. B., Dixit, M., Zepeda, J. C., Grueter, B. A. & Dugan, L. L. Robust expression of functional NMDA receptors in human induced pluripotent stem cell-derived neuronal cultures using an accelerated protocol. Front. Mol. Neurosci. 14, 777049 (2021).
Neagoe, I. et al. The GluN2B subunit represents a major functional determinant of NMDA receptors in human induced pluripotent stem cell-derived cortical neurons. Stem Cell Res. 28, 105–114 (2018).
Keefe, M. G., Steyert, M. R. & Nowakowski, T. J. Lineage-resolved atlas of the developing human cortex. Nature 647, 194–202 (2025).
Strehlow, V. et al. GRIN2A-related disorders: genotype and functional consequence predict phenotype. Brain 142, 80–92 (2019).
Verhaeghe, R., Elía-Zudaire, O., Escamilla, S., Sáez-Valero, J. & Pérez-Otaño, I. No evidence for cognitive decline or neurodegeneration in strain-matched Grin3a knockout mice. Alzheimers Dement. 19, 4264–4266 (2023).
Vyklicky, V. et al. Surface expression, function, and pharmacology of disease-associated mutations in the membrane domain of the human GluN2B subunit. Front. Mol. Neurosci. 11, 110 (2018).
Steg, L. C. et al. Novel epigenetic clock for fetal brain development predicts prenatal age for cellular stem cell models and derived neurons. Mol. Brain 14, 98 (2021).
Pasca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Gordon, A. et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 24, 331–342 (2021).
Alam El Din, D. M. et al. Human neural organoid microphysiological systems show the building blocks necessary for basic learning and memory. Commun. Biol. 8, 1237 (2025).
Harris, A. Z. & Pettit, D. L. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J. Physiol. 584, 509–519 (2007).
Escamilla, S., Sáez-Valero, J. & Cuchillo-Ibáñez, I. NMDARs in Alzheimer’s disease: Between synaptic and extrasynaptic membranes. Int. J. Mol. Sci. 25, 10220 (2024).
Li, S. et al. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 31, 6627–6638 (2011).
Snyder, E. M. et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat. Neurosci. 8, 1051–1058 (2005).
Tu, W. et al. DAPK1 Interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell 140, 222–234 (2010).
Wu, Q. J. & Tymianski, M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol. Brain 11, 15 (2018).
Levine, M. S., Cepeda, C. & André, V. M. Location, location, location: Contrasting roles of synaptic and extrasynaptic NMDA receptors in Huntington’s disease. Neuron 65, 145–147 (2010).
Milnerwood, A. J. et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron 65, 178–190 (2010).
Escamilla, S. et al. Synaptic and extrasynaptic distribution of NMDA receptors in the cortex of Alzheimer’s disease patients. Alzheimer’s Dementia 20, 8231–8245 (2024).
Parsons, M. P. & Raymond, L. A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 82, 279–293 (2014).
Yu, S. P., Jiang, M. Q., Shim, S. S., Pourkhodadad, S. & Wei, L. Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: Implications for preventive treatments of ischemic stroke and late-onset Alzheimer’s disease. Mol. Neurodegener. 18, 43 (2023).
Corbel, C., Hernandez, I., Wu, B. & Kosik, K. S. Developmental attenuation of N-methyl-D-aspartate receptor subunit expression by microRNAs. Neural Dev. 10, 20 (2015).
Rodenas-Ruano, A., Chávez, A. E., Cossio, M. J., Castillo, P. E. & Zukin, R. S. REST-dependent epigenetic remodeling promotes the developmental switch in synaptic NMDA receptors. Nat. Neurosci. 15, 1382–1390 (2012).
Ferreira, J. S. et al. Co-agonists differentially tune GluN2B-NMDA receptor trafficking at hippocampal synapses. Elife 6, e25492 (2017).
Groc, L. et al. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein reelin. J. Neurosci. 27, 10165–10175 (2007).
Matta, J. A., Ashby, M. C., Sanz-Clemente, A., Roche, K. W. & Isaac, J. T. R. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 70, 339–351 (2011).
Nolt, M. J. et al. EphB controls NMDA receptor function and synaptic targeting in a subunit-specific manner. J. Neurosci. 31, 5353–5364 (2011).
Rzechorzek, N. M. et al. Hypothermic preconditioning reverses tau ontogenesis in human cortical neurons and is mimicked by protein phosphatase 2A inhibition. EBioMedicine 3, 141–154 (2016).
Zhang, Y., Li, P., Feng, J. & Wu, M. Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol. Sci. 37, 1039–1047 (2016).
Lam, R. S., Töpfer, F. M., Wood, P. G., Busskamp, V. & Bamberg, E. Functional maturation of human stem cell-derived neurons in long-term cultures. PLoS ONE 12, e0169506 (2017).
Zhang, Y. et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 (2013).
Nakamura, T. et al. Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons. J. Neurosci. 20, 8365–8376 (2000).
McLeod, J. R., Shen, M., Kim, D. J. & Thayer, S. A. Neurotoxicity mediated by aberrant patterns of synaptic activity between rat hippocampal neurons in culture. J. Neurophysiol. 80, 2688–2698 (1998).
D’Andrea, T., Benedetti, M. C., Monaco, L., Rosa, A. & Fucile, S. Selective reduction of Ca2+ entry through the human nmda receptor: A quantitative study by simultaneous Ca2+ and Na+ imaging. Mol. Neurobiol. 61, 5841–5850 (2024).
Galiakberova, A. A. et al. IPSC-derived human neurons with GCaMP6s expression allow in vitro study of neurophysiological responses to neurochemicals. Neurochem. Res. 47, 952–966 (2022).
Pruunsild, P., Bengtson, C. P. & Bading, H. Networks of cultured iPSC-derived neurons reveal the human synaptic activity-regulated adaptive gene program. Cell Rep. 18, 122–135 (2017).
Hernandez, A. & Stohn, J. P. The type 3 deiodinase: Epigenetic control of brain thyroid hormone action and neurological function. Int. J. Mol. Sci. 19, 1804 (2018).
Mo, C.-F. et al. Loss of non-coding RNA expression from the DLK1-DIO3 imprinted locus correlates with reduced neural differentiation potential in human embryonic stem cell lines. Stem Cell Res. Ther. 6, 1 (2015).
Salas-Lucia, F., Escamilla, S., Bianco, A. C., Dumitrescu, A. & Refetoff, S. Impaired T3 uptake and action in MCT8-deficient cerebral organoids underlie Allan-Herndon-Dudley syndrome. JCI Insight 9, e174645 (2024).
Salas-Lucia, F. et al. Axonal T3 uptake and transport can trigger thyroid hormone signaling in the brain. Elife 12, e82683 (2023).
Salas-Lucia, F. Mapping Thyroid Hormone Action in the Human Brain. Thyroid® 34(815–826), 815 (2024).
Montalbán-Loro, R. et al. Dlk1 dosage regulates hippocampal neurogenesis and cognition. Proc. Natl. Acad. Sci. 118, e2015505118 (2021).
Ferrón, S. R. et al. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature 475, 381–385 (2011).
Acknowledgements
We thank Prof. Juan Lerma for generously providing access to his laboratory facilities for the patch clamp experiments, for his assistance with data analysis and for his insightful contributions that greatly enriched this work.
Funding
This work was supported by grants from the Fondo de Investigaciones Sanitarias (PI22/01329, co-funded by the Fondo Europeo de Desarrollo Regional, FEDER “Investing in your future”), CIBERNED (Instituto de Salud Carlos III, Spain), the Instituto de Investigación Sanitaria y Biomédica de Alicante (Isabial), Direcció General de Ciència i Investigació, Generalitat Valenciana (CIAICO/2024/313) and “Severo Ochoa” Program for Centers of Excellence in R&D (CEX2021-001165-S). SE is supported by a PFIS fellowship from the ISC‐III. CAG is supported by a predoctoral contract. (PRE2022-104182) funded by the Agencia Estatal de Investigación (AEI) and the Ministerio de Ciencia e Innovación (MCIN) under the Plan Estatal de I + D+I 2021–2023, co-funded by the European Social Fund (FSE). FS-L is supported by University of Chicago start-up funds. AVP is supported by the Spanish Agency of Research (AEI) under the grants PID2022-136741NB-100 and by the Generalitat Valenciana through the program PROMETEO/2019/014 and CIPROM/2022/08 to Juan Lerma. EDLP and FAP are supported by grant PID2022-140961OB-100 (MCIN/AEI/https://doi.org/10.13039/501100011033), grant PROMETEO/2021/031 (Generalitat Valenciana Government) and “Severo Ochoa” Program for Centers of Excellence in R&D (CEX2021-001165-S). HZ is a Wallenberg Scholar and a Distinguished Professor at the Swedish Research Council supported by grants from the Swedish Research Council (#2023 − 00356, #2022 − 01018 and #2019–02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, and Swedish State Support for Clinical Research (#ALFGBG-71320).
Author information
Authors and Affiliations
Contributions
SE and CAG performed the iPSC cultures and differentiation, FACS, ICQ, WB and RT-qPCR and prepared Figs. 1–2. HZ donates the cells and revised the manuscript. MACG participated in the design and culture of iPSC. EDLP and FAP performed the Fura-2 studies and prepared Fig. 4. AVP performed the patch-clamp studies and prepared Fig. 3 and Supp Fig. 2. FSL and SE performed the RNAseq studies and prepared Supp Fig. 1 and Supp Excells. ICI and SE wrote the main manuscript text. ICI and JSV revised it substantively. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing of interests
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Enigma, LabCorp, Merck Sharp & Dohme, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, ScandiBio Therapeutics AB, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures sponsored by Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, LabCorp, Lilly, Novo Nordisk, Oy Medix Biochemica AB, Roche, and WebMD, is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, and is a shareholder of MicThera (outside submitted work).
Ethical approval
The human cells (iPSCs) were a gift and the original source of the cells, the laboratory of Dr Henrik Zetterberg, confirm that there was initial ethical approval for collection of human cells, and that the donors had signed informed consent. The authors declare that they have not used AI-generated work in this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Escamilla, S., Avilés-Granados, C., Peralta, F.A. et al. GluN2A-mediated currents and calcium signal in human iPSC-derived neurons. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38482-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38482-y