Abstract
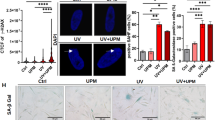
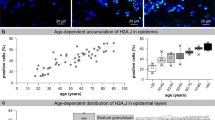
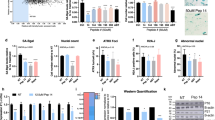
Targeting cellular senescence presents a promising approach to slow visible skin aging and promote tissue repair. However, most preclinical models fail to capture the full architecture of human skin or accommodate diverse skin types, limiting their translational relevance. To address this gap, we developed a controlled ex vivo human skin explant platform using freshly acquired tissues from donors of varying ages and Fitzpatrick skin types. This model applies standardized UVA and UVB doses to induce reproducible photodamage, enabling the assessment of both preventative and reparative effects of topical treatments. The results showed that ND-ZnO and NAC reduced levels of p16^INK4a and p53, which are key biomarkers measuring cellular senescence; ND-ZnO and exosomes lowered IL-1β expression, which is a biomarker measuring inflammation. Histological analysis confirmed these findings, with ND-ZnO-treated skins preserved epidermal structure, reduced inflammatory features, and maintained dermal collagen organization. We then conducted a four-week single-patient case study using the same ND-ZnO formulation. Visible improvements in redness, pigmentation, and texture were observed, aligned with the molecular and histological changes seen ex vivo. These findings suggested that the ex vivo platform has the potential to be used as a more inclusive, human-relavent model for evaluating and quantifying the anti-aging efficacies of topical treatments across diverse skin types and age groups.
Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article. Additional raw data and analysis scripts are available from the corresponding author upon reasonable request.
References
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell Jun. 6 (6), 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039 (2013).
Campisi, J. D’Adda Di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev. Mol. Cell. Biol. 8 (9), 729–740. https://doi.org/10.1038/nrm2233 (2007).
Piipponen, M., Riihilä, P., Nissinen, L. & Kähäri, V. M. The role of p53 in progression of cutaneous squamous cell carcinoma. Cancers (Basel) 7 (18). https://doi.org/10.3390/cancers13184507 (2021).
Rufini, A., Tucci, P., Celardo, I. & Melino, G. Senescence and aging: the critical roles of p53. Oncogene 24 (43), 5129–5143. https://doi.org/10.1038/onc.2012.640 (2013).
Lyons, C. E. et al. Chronic social stress induces p16-mediated senescent cell accumulation in mice. Nat. Aging.. 5 (1), 48–64. https://doi.org/10.1038/s43587-024-00743-8 (2025).
Baker, D. J. et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 11 (7589), 184–189. https://doi.org/10.1038/nature16932 (2016).
Ressler, S. et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 5 (5), 379–389. https://doi.org/10.1111/j.1474-9726.2006.00231.x (2006).
Casas, J. W. et al. In vitro human skin irritation test for evaluation of medical device extracts. Toxicol. In Vitro. 27 (8), 2175–2183. https://doi.org/10.1016/j.tiv.2013.08.006 (2013). 2013/12/01.
Larson, P. J., Chong, D., Fleming, E. & Oh, J. Challenges in developing a human model system for skin Microbiome research. J. Invest. Dermatol.. 141 (1), 228–231e4. https://doi.org/10.1016/j.jid.2020.05.096 (2021).
Neil, J. E., Brown, M. B. & Williams, A. C. Human skin explant model for the investigation of topical therapeutics. Sci. Rep. 3 (1), 21192. https://doi.org/10.1038/s41598-020-78292-4 (2020).
Portugal-Cohen, M., Cohen, D., Kohen, R. & Oron, M. Exploitation of alternative skin models from academia to industry: proposed functional categories to answer needs and regulation demands. Front. Physiol. 14, 1215266. https://doi.org/10.3389/fphys.2023.1215266 (2023).
Sanabria-de la Torre, R., Fernández-González, A., Quiñones-Vico, M. I., Montero-Vilchez, T. & Arias-Santiago, S. Bioengineered skin intended as in vitro model for Pharmacosmetics, skin disease study and environmental skin impact analysis. Biomedicines 31 (11). https://doi.org/10.3390/biomedicines8110464 (2020).
Mathes, S. H., Ruffner, H. & Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Del. Rev.. 81–102. https://doi.org/10.1016/j.addr.2013.12.006 (2014).
Tellkamp, F. et al. Transgenic Mouse Technology in Skin Biology: Generation of Knockin Mice. J. Investig. Dermatol.. https://doi.org/10.1038/jid.2014.434 (2014).
Wang, H. et al. An ex vivo model of medical device-mediated bacterial skin translocation. Sci. Rep.. 11(1):5746 https://doi.org/10.1038/s41598-021-84826-1 (2021).
Cai, Y. et al. A critical role of the IL-1β-IL-1R signaling pathway in skin inflammation and psoriasis pathogenesis. J. Invest. Dermatol.. 139 (1), 146–156. https://doi.org/10.1016/j.jid.2018.07.025 (2019).
Jensen, L. E. Targeting the IL-1 family members in skin inflammation. Curr. Opin. Investig. Drugs.. 11 (11), 1211–1220 (2010).
Zhang, X. D. et al. Enhanced skin regeneration and therapeutic delivery using novel diamond-augmented zinc oxide. J. Cosmet. Dermato.. 31 https://doi.org/10.1111/jocd.16508 (2024).
Janeczek, M. et al. The potential uses of N-acetylcysteine in dermatology: A review. J. Clin. Aesthet. Dermatol.. 12 (5), 20–26 (2019).
Prasai, A., Jay, J. W., Jupiter, D., Wolf, S. E. & El Ayadi, A. Mar. Role of Exosomes in Dermal Wound Healing: A Systematic Review. J. Invest. Dermatol.. ;142(3 Pt A):662–678 e8. https://doi.org/10.1016/j.jid.2021.07.167 (2022).
Xiong, M. et al. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol. Res.. 166, 105490. https://doi.org/10.1016/j.phrs.2021.105490 (2021).
Saguie, B. O., Martins, R. L., Fonseca AdSd, Romana-Souza, B. & Monte-Alto-Costa, A. An ex vivo model of human skin Photoaging induced by UVA radiation compatible with summer exposure in Brazil. J. Photochem. Photobiol., B. 221, 112255. https://doi.org/10.1016/j.jphotobiol.2021.112255 (2021).
Mayangsari, E., Mustika, A., Nurdiana, N. & Samad, N. A. Comparison of UVA vs UVB Photoaging rat models in Short-term exposure. Med. Arch. 78 (2), 88–91. https://doi.org/10.5455/medarh.2024.78.88-91 (2024).
Cai, Y. et al. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res.. 30(7), 574–589.https://doi.org/10.1038/s41422-020-0314-9 (2020).
Wang, F. et al. Downregulated TFPI2 accelerates skin aging by repressing the cell cycle through phosphoinositide 3-Kinase/Protein kinase B/CDC6 pathway. J. Invest. Dermatol.. 145 (8), 1910–1920e7. https://doi.org/10.1016/j.jid.2024.11.028 (2025).
Bai, X., Yan, J. & Gilchrest, B. A. Next-Generation zinc Oxide-Based sunscreens: molecular characteristics and advantages. J. Invest. Dermatol.. 144 (2), 430–434e1. https://doi.org/10.1016/j.jid.2023.07.020 (2024).
Berridge, B. R. M. V. et al. Non-proliferative and proliferative lesions of the cardiovascular system of the rat and mouse. Toxicol. Pathol. 29 (3 Suppl), 1S–47S. https://doi.org/10.1293/tox.29.3S-1 (2016).
Thoolen, B. et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 38 (7 Suppl), 5S–81S. https://doi.org/10.1177/0192623310386499 (2010).
Funding
This work was supported by internal research funding from B.A.I. Biosciences.
Author information
Authors and Affiliations
Contributions
X.D.Z., X.B., and C.T. conceived and designed the study. X.D.Z., N.A., and E.R. performed the experiments. X.D.Z. analyzed the data, and J.T. contributed to histological data interpretation. All figures were prepared by X.D.Z. The manuscript was drafted by X.D.Z., N.A., E.R., C.T., and X.B., and reviewed and edited by X.B., C.T., J.T., and X.D.Z.
Corresponding author
Ethics declarations
Competing interests
X.D.Z, C.T., and X.B. are employees of B.A.I. Biosciences. X.D.Z. and X.B. are named inventors on a patent application related to the nanodiamond zinc oxide formulation described in this study, which is solely owned by B.A.I. Biosciences. The remaining authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X.D., Atalla, N., Rodriguez, E. et al. Development of a controlled ex vivo human skin platform for quantitative evaluation of age-related functional biomarkers following application of topical treatments. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38877-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38877-x