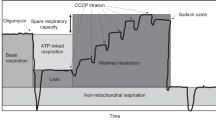
Abstract
Sepsis is associated with high rates of multiorgan failure and mortality. Altered mitochondrial function is an essential component of the early sepsis syndrome. However, its progression over time in peripheral blood mononuclear cells (PBMCs) is thus far unclear. Our purpose was to investigate this in the early phase of sepsis in ICU patients. A single-centre prospective observational cohort study was conducted in sepsis patients and compared with age- and sex-matched controls. Mitochondrial function was measured in PBMCs thrice during the first ICU week. RT-qPCR was used for semi-quantitative analysis of expression of genes involved in oxidative phosphorylation. Secondary endpoints included associations between mitochondrial function and (I) sepsis severity and (II) clinical outcomes, including 3-month mortality. Basal, ATP-linked, maximal and proton leak associated respiration were increased in sepsis patients (n = 25) compared to matched controls (n = 24) at all time points. This was associated with increased expression of SDBH (complex II) and ATP5F1A (complex V). Increased basal respiration was associated with 3-month mortality (HR 3.794, 95% CI 1.018–14.149, p = 0.047). No differences were observed in other secondary outcomes. PBMC mitochondria were shown to have an increased respiratory rate during the first week of sepsis. Moreover, a progressive increase was negatively associated with 3-month survival.
Similar content being viewed by others
Data availability
The dataset supporting the conclusions of this article is available upon reasonable request from the corresponding author.
Abbreviations
- ADP:
-
Adenine diphosphate
- ATP:
-
Adenine triphosphate
- APACHE II:
-
Acute physiology and chronic health evaluation II
- BMI:
-
Body mass index
- CCCP:
-
Carbonyl cyanide m-chlorophenylhydrazone
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- FEV1:
-
First second forced expiration
- FVC:
-
Forced vital capacity
- ICU:
-
Intensive care unit
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- LMR:
-
Lymphocyte-monocyte ratio
- LOS:
-
Length of stay
- mNUTRIC:
-
Modified nutrition risk in critically ill score
- OXPHOS:
-
Oxidative phosphorylation system
- PBMC:
-
Peripheral blood mononuclear cells
- RCR:
-
Respiratory control ratio
- SARS-CoV-2:
-
Severe acute respiratory coronavirus 2
- SD:
-
Standard deviation
- SOFA:
-
Sequential organ failure assessment score
- TCA:
-
Tricarboxylic acid
- VIF:
-
Variation inflation factor
- WUR:
-
Wageningen University and Research
- ZGV:
-
Ziekenhuis Gelderse Vallei (Gelderse Vallei hospital)
References
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315(8), 801–810 (2016).
Bauer, M. et al. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019-results from a systematic review and meta-analysis. Crit. Care 24(1), 239 (2020).
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 395(10219), 200–211 (2020).
Yende, S. et al. Long-term quality of life among survivors of severe sepsis: Analyses of two international trials. Crit. Care Med. 44(8), 1461–1467 (2016).
Wischmeyer, P. E. & San-Millan, I. Winning the war against ICU-acquired weakness: New innovations in nutrition and exercise physiology. Crit. Care 19(Suppl 3), S6 (2015).
Belikova, I. et al. Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit. Care Med. 35(12), 2702–2708 (2007).
Japiassú, A. M. et al. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5’-triphosphate synthase activity. Crit. Care Med. 39(5), 1056–1063 (2011).
Garrabou, G. et al. The effects of sepsis on mitochondria. J. Infect. Dis. 205(3), 392–400 (2012).
Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 5(1), 66–72 (2014).
Thiessen, S. E., Van den Berghe, G. & Vanhorebeek, I. Mitochondrial and endoplasmic reticulum dysfunction and related defense mechanisms in critical illness-induced multiple organ failure. Biochim. Biophys. Acta Mol. Basis Dis. 1863(10), 2534–2545 (2017).
Brand, M. D. & Nicholls, D. G. Assessing mitochondrial dysfunction in cells. Biochem. J. 435(2), 297–312 (2011).
Supinski, G. S., Schroder, E. A. & Callahan, L. A. Mitochondria and critical illness. Chest 157(2), 310–322 (2020).
Singer, M. Critical illness and flat batteries. Crit. Care. 21(Suppl 3), 309 (2017).
Exline, M. C. & Crouser, E. D. Mitochondrial mechanisms of sepsis-induced organ failure. Front Biosci. 13, 5030–5041 (2008).
Brealey, D. et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360(9328), 219–223 (2002).
Gründler, K. et al. Platelet mitochondrial membrane depolarization reflects disease severity in patients with sepsis and correlates with clinical outcome. Crit. Care 18(1), R31 (2014).
Kleiveland, C. R. Peripheral blood mononuclear cells. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models [Internet] (eds Verhoeckx, K. et al.) (Springer, Cham, 2015). https://doi.org/10.1007/978-3-319-16104-4_15.
Jang, D. H. et al. Alterations in mitochondrial function in blood cells obtained from patients with sepsis presenting to an emergency department. Shock 51(5), 580–584 (2019).
Sjövall, F., Morota, S., Persson, J., Hansson, M. J. & Elmér, E. Patients with sepsis exhibit increased mitochondrial respiratory capacity in peripheral blood immune cells. Crit. Care 17(4), R152 (2013).
Clere-Jehl, R. et al. Septic shock alters mitochondrial respiration of lymphoid cell-lines and human peripheral blood mononuclear cells: The role of plasma. Shock 51(1), 97–104 (2019).
Silaidos, C. et al. Sex-associated differences in mitochondrial function in human peripheral blood mononuclear cells (PBMCs) and brain. Biol. Sex Differ. 9(1), 34 (2018).
Li, S., Li, X., Jiang, S., Wang, C. & Hu, Y. Identification of sepsis-associated mitochondrial genes through RNA and single-cell sequencing approaches. BMC Med. Genom. 17(1), 120 (2024).
Nucci, L. A. et al. Expression of genes belonging to the interacting TLR cascades, NADPH-oxidase and mitochondrial oxidative phosphorylation in septic patients. PLoS ONE 12(2), e0172024 (2017).
Gerratana, L. et al. Biologically driven cut-off definition of lymphocyte ratios in metastatic breast cancer and association with exosomal subpopulations and prognosis. Sci. Rep. 10(1), 7010 (2020).
Kramer, P. A., Ravi, S., Chacko, B., Johnson, M. S. & Darley-Usmar, V. M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox Biol. 2, 206–210 (2014).
Fredriksson, K. et al. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS ONE 3(11), e3686 (2008).
Fredriksson, K. et al. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am. J. Physiol. Endocrinol. Metab. 291(5), E1044–E1050 (2006).
Jeger, V., Djafarzadeh, S., Jakob, S. M. & Takala, J. Mitochondrial function in sepsis. Eur. J. Clin. Invest. 43(5), 532–542 (2013).
Nedel, W., Deutschendorf, C. & Portela, L. V. C. Sepsis-induced mitochondrial dysfunction: A narrative review. World J. Crit. Care Med. 12(3), 139–152 (2023).
Hotchkiss, R. et al. Sepsis and septic shock. Nat. Rev. Dis. Primers 30(2), 16045 (2016).
Drewry, A. M. et al. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 42(5), 383–391 (2014).
Nedel, W., Henrique, L. R. & Portela, L. V. C. Why should lymphocytes immune profile matter in sepsis?. World J. Crit. Care Med. 14(2), 98791 (2025).
Sun, Z. et al. Sepsis endotypes defined by lymphocyte thresholds and inflammation inform precision immunomodulation. MedComm 7(1), e70561 (2026).
Acknowledgements
We owe thanks to the following people for their valuable contributions to data collection and the writing of preliminary reports: Monique Daanje, Rosan van den Boogaard, Demi Vogels, Laura Willemse, Marloes Snijder, Max van den Bergh, Julia Boeré, Susana Mittmann, Panagiotis Vlachogiannis, Marieke Krüger, Rebeka Darmati, Viditha Venkatramu and Lisa Kleverwal. We thank all participants of the study, as well as the MSc students and research nurses who aided in data collection.
Funding
This research was funded by the Research Foundation of the Intensive Care of Gelderse Vallei Hospital, Ede, The Netherlands. Any article processing charges will be put forth by Wageningen University & Research.
Author information
Authors and Affiliations
Contributions
HM and RSB contributed equally to data collection, data analysis and interpretation, and writing and revising of the manuscript. AvN, SG, and JdJ contributed to the conception of the study, data collection, analysis, interpretation, and revision of the manuscript. AW contributed to data collection and interpretation. AvZ contributed to the conception of the study, data interpretation and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Prof. Dr. Van Zanten reported receiving honoraria for advisory board meetings, lectures, research grants, and travel expenses from Abbott, AOP Pharma, Baxter, Danone-Nutricia, Dutch Medical Food, Fresenius Kabi, GE Healthcare, Medcaptain, Nestle, PAION and Rousselot. The other authors have nothing to declare.
Ethics approval and consent to participate
The study was approved by the Medical Ethical Committee of Wageningen University (METC-WUR, which was incorporated in the METC Oost-Nederland in 2021, dossier no. 2021–13011) and the assessment Committee for Scientific Research of ZGV (dossier no. 1801–004). The protocol was registered in the Netherlands Trial Register (number NTR6969) and was made available through the International Clinical Trial Registry Platform (NL5918). Patients were enrolled after signing the informed consent by the patient or legal representative.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Moonen, H.P.F.X., Slingerland-Boot, R., de Jong, J.C.B.C. et al. Progression of peripheral blood mononuclear cell mitochondrial function during the early phase of sepsis in intensive care unit patients. Sci Rep (2026). https://doi.org/10.1038/s41598-026-39202-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-39202-2