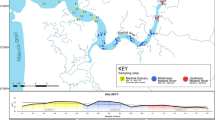
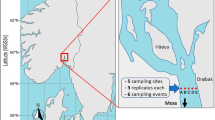
Abstract
Urban wetlands provide crucial ecosystem services but are increasingly threatened by urbanization. In Macao, a densely populated city on the Pearl River Estuary, roughly half of the historical wetland habitats have been lost, yet they remain vital for the East Asian–Australasian Flyway. To assess biodiversity in the remaining wetlands, this study applied environmental DNA (eDNA) metabarcoding targeting 12S rRNA, 18S rRNA, and COI genes with a primary focus on fish and invertebrates. The results revealed 85 fish, 298 invertebrate and 9 non-fish chordate species, including 18 non-indigenous fish and several invertebrates. The communities were highly site-specific, showing clear distinctions between inland and coastal wetlands, but non-indigenous fish were widespread, reflecting strong anthropogenic pressure. Moreover, while not observed in fish, coastal invertebrate communities showed strong seasonal turnover. Nevertheless, 56% of COI-derived ESVs could only be assigned to higher taxonomic levels, suggesting substantial diversity remains uncharacterized due to incomplete reference databases. Collectively, these findings demonstrate how fragmentation and seasonal dynamics shape biodiversity differently across taxonomic groups. This study establishes the first comprehensive eDNA baseline for Macao’s wetlands, highlighting the need to expand local reference databases and integrate molecular techniques with traditional surveys to improve monitoring and conservation of urban ecosystems.
Similar content being viewed by others
Data availability
Raw sequencing data generated in this study have been deposited in the NCBI Sequence Read Archive (SRA) under BioProject **PRJNA1344804 (** https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1344804). Supplementary Information provides full lists of detected taxa, including taxonomic identification, primers, and the sites where each taxon was detected, as well as tables summarizing raw reads, merged reads, and reads retained after quality filtering for each library.
References
Alikhani, S., Nummi, P. & Ojala, A. Urban wetlands: A review on ecological and cultural values. Water 13 https://doi.org/10.3390/w13223301 (2021).
Bhowmik, S. Ecological and economic importance of wetlands and their vulnerability: A review. Research Anthology Ecosyst. Conserv. Preserving Biodiversity, 11–27 (2022).
Datri, L. A., Lopez, M., Buchter, S., Pazcel, E. M. & Gandini, M. Functional urban wetlands in dysfunctional cities. Curr. Landsc. Ecol. Rep. 9, 21–30 (2024).
Kumar, S. et al. Constructed wetland management in urban catchments for mitigating floods. Stoch. Env. Res. Risk Assess. 35, 2105–2124 (2021).
McInnes, R. J. Recognising wetland ecosystem services within urban case studies. Mar. Freshw. Res. 65, 575–588 (2013).
Mitsch, W. J., Zhang, L., Griffiths, L. N. & Bays, J. Contrasting two urban wetland parks created for improving habitat and downstream water quality. Ecol. Eng. 192, 106976 (2023).
Rome, M. et al. Application of floating wetlands for the improvement of degraded urban waters: findings from three multi-year pilot-scale installations. Sci. Total Environ. 877, 162669 (2023).
Kulik, M. et al. Half a century of wetland degradation: the present state and trends of changes in Western Polesie-Long-term wetland degradation. Global Ecol. Conserv. 56, e03324 (2024).
Robertson, H. et al. Global Wetland Outlook. https://doi.org/10.69556/GWO-2025-eng (2025)
Ballut-Dajud, G. A. et al. Factors affecting wetland loss: A review. Land 11, 434 (2022).
Huang, X., Wu, Z., Zhang, Q. & Cao, Z. How to measure wetland destruction and risk: wetland damage index. Ecol. Ind. 141, 109126 (2022).
Chu, N. et al. Hydrodynamical transport structure and lagrangian connectivity of circulations in the Pearl river estuary. Front. Mar. Sci. 9, 996551 (2022).
Wang, H. et al. Mangrove loss and gain in a densely populated urban estuary: lessons from the Guangdong-Hong Kong-Macao greater Bay area. Front. Mar. Sci. 8, 693450 (2021).
Yang, X. et al. Restoration of urban waterbird diversity: A case study of the construction of a waterbird ecological corridor in the Guangdong-Hong Kong-Macao greater Bay Area, Southern China. Global Ecol. Conserv. 39, e02277 (2022).
Yi, G., Fenzhen, S., Xiaoyu, S., Zhenshan, X. & Yawen, H. 429–432 (IEEE).
Nanwei, L. Geografia natural de Macau. (1992).
Quelhas, P. et al. Geology of the Macao special administrative region (China). J. Maps. 17, 257–267 (2021).
Carvalho, J. Taipa Coloane: Macau Da Outra Banda (Câmara Municipal das Ilhas, 1998).
Statistics and Census Service (DSEC). Demographic Statistics for the First Three Quarters of 2025 (Macao SAR Government, 2025).
Macau & Land Public Works and Transport Bureau (DSSOPT). Macao Urban Master Plan (2020–2040) (Macao SAR Government, 2021).
Kaller, M. D., Kelso, W. E. & Trexler, J. C. In Wetland Techniques: Volume 2: Organisms 197–263 (Springer, 2013).
Coleman, R., Raadik, T., Pettigrove, V. & Hoffmann, A. Taking advantage of adaptations when managing threatened species within variable environments: The case of the Dwarf galaxias, Galaxiella pusilla (Teleostei, Galaxiidae). Mar. Freshw. Res. 68, 175–186 (2016).
King, A. J., George, A., Buckle, D. J., Novak, P. A. & Fulton, C. J. Efficacy of remote underwater video cameras for monitoring tropical wetland fishes. Hydrobiologia 807, 145–164 (2018).
Fediajevaite, J., Priestley, V., Arnold, R. & Savolainen, V. Meta-analysis shows that environmental DNA outperforms traditional surveys, but warrants better reporting standards. Ecol. Evol. 11, 4803–4815 (2021).
Rees, H. C., Maddison, B. C., Middleditch, D. J., Patmore, J. R. & Gough, K. C. The detection of aquatic animal species using environmental DNA–a review of eDNA as a survey tool in ecology. J. Appl. Ecol. 51, 1450–1459 (2014).
Martin, M. E. et al. Conservation of rare and cryptic species: Challenges of uncertainty and opportunities for progress. Conserv. Sci. Pract. 4, e12809 (2022).
Lindenmayer, D. et al. A checklist of attributes for effective monitoring of threatened species and threatened ecosystems. J. Environ. Manage. 262, 110312 (2020).
Banerjee, P. et al. Environmental DNA analysis as an emerging non-destructive method for plant biodiversity monitoring: a review. AoB Plants. 14, plac031 (2022).
Larson, E. R. et al. From eDNA to citizen science: emerging tools for the early detection of invasive species. Front. Ecol. Environ. 18, 194–202 (2020).
Yoon, H. J., Seo, J. H., Shin, S. H., Abedlhamid, M. A. & Pack, S. P. Bioinformation and monitoring technology for environmental DNA analysis: A review. Biosensors 15, 494 (2025).
Zhang, S., Zhao, J. & Yao, M. Urban landscape-level biodiversity assessments of aquatic and terrestrial vertebrates by environmental DNA metabarcoding. J. Environ. Manage. 340, 117971 (2023).
Liu, Y. S. et al. Investigation of fish diversity in lake Taihu based on eDNA metabarcoding. (2023). https://doi.org/10.7524/AJE.1673-5897.20230717002
Deiner, K. et al. Environmental DNA metabarcoding: transforming how we survey animal and plant communities. Mol. Ecol. 26, 5872–5895 (2017).
Nhat, N. H., Saito, M., Hamada, M. & Onodera, S. -i. Evaluation of the effects of environmental factors on seasonal variations in fish diversity on a coastal Island in Western Japan. Environ. (Basel Switzerland). 11, 60. https://doi.org/10.3390/environments11030060 (2024).
Stoeck, T. et al. Multiple marker parallel Tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol. Ecol. 19, 21–31. https://doi.org/10.1111/j.1365-294X.2009.04480.x (2010).
Environmental Protection Bureau (DSPA). Zonas Ecológicas do Cotai. (2024).
Nelson, S. M., Roline, R. A., Thullen, J. S., Sartoris, J. J. & Boutwell, J. E. Invertebrate assemblages and trace element bioaccumulation associated with constructed wetlands. Wetlands 20, 406–415 (2000).
Kumari, D. Paul Dilip Kumar. Assessing the role of bioindicators in freshwater ecosystem. J. Interdisciplinary Cycle Res. 12, 17 (2020).
Ferreira, A. O. et al. Multi-marker DNA metabarcoding for precise species identification in ichthyoplankton samples. Sci. Rep. 14, 19772–19714. https://doi.org/10.1038/s41598-024-69963-7 (2024).
Leite, B. R., Vieira, P. E., Troncoso, J. S. & Costa, F. O. Comparing species detection success between molecular markers in DNA metabarcoding of coastal macroinvertebrates. Metabarcoding Metagenomics. 5, 249–260. https://doi.org/10.3897/mbmg.5.70063 (2021).
Zhang, S., Zhao, J., Yao, M. & Gilbert, M. A comprehensive and comparative evaluation of primers for metabarcoding eDNA from fish. Methods Ecol. Evol. 11, 1609–1625. https://doi.org/10.1111/2041-210X.13485 (2020).
Fais, M. et al. Small-scale Spatial variation of meiofaunal communities in Lima estuary (NW Portugal) assessed through metabarcoding. Estuar. Coast. Shelf Sci. 238, 106683. https://doi.org/10.1016/j.ecss.2020.106683 (2020).
Hebert, P. D. N., Cywinska, A., Ball, S. L. & deWaard, J. R. Biological identifications through DNA barcodes. Proc. Royal Soc. B: Biol. Sci. 270, 313–321. https://doi.org/10.1098/rspb.2002.2218 (2003).
Lavrador, A. S. et al. Comprehensive DNA metabarcoding-based detection of non-indigenous invertebrates in recreational marinas through a multi-substrate approach. Mar. Environ. Res. 200, 106660 (2024).
van der Reis, A. L., Beckley, L. E., Olivar, M. P. & Jeffs, A. G. Nanopore short-read sequencing: A quick, cost‐effective and accurate method for DNA metabarcoding. Environ. DNA (Hoboken N J). 5, 282–296. https://doi.org/10.1002/edn3.374 (2023).
Miya, M. et al. MiFish, a set of universal PCR primers for metabarcoding environmental DNA from fishes: detection of more than 230 subtropical marine species. Royal Soc. Open. Sci. 2, 150088 (2015).
I.A.M. Macau Municipal Affairs Bureau, https://www.iam.gov.mo/e/default/).
Perkin, J. S., Gido, K. B. & Nilsson, C. Fragmentation alters stream fish community structure in dendritic ecological networks. Ecol. Appl. 22, 2176–2187. https://doi.org/10.1890/12-0318.1 (2012).
SchÜCkel, U. & Kroncke, I. Temporal changes in intertidal macrofauna communities over eight decades: A result of eutrophication and climate change. Estuar. Coast. Shelf Sci. 117, 210–218. https://doi.org/10.1016/j.ecss.2012.11.008 (2013).
Beisel, J. N., Usseglio-Polatera, P. & Moreteau, J. C. The Spatial heterogeneity of a river bottom: a key factor determining macroinvertebrate communities. Hydrobiologia 422–423, 163–171. https://doi.org/10.1023/A:1017094606335 (2000).
Whitfield, A. K. & Elliott, M. Fishes as indicators of environmental and ecological changes within estuaries: a review of progress and some suggestions for the future. J. Fish Biol. 61, 229–250. https://doi.org/10.1006/jfbi.2002.2079 (2002).
Lai, H. et al. Seasonal variation in the functional structure of demersal fish communities and response to the environmental changes in the Pearl river Estuary, China. Ecol. Ind. 144, 109525. https://doi.org/10.1016/j.ecolind.2022.109525 (2022).
Li, Z. et al. A comparison of seasonal composition and structure of fish community between environmental DNA technology and gillnetting in the Pearl river Estuary, China. Ecol. Ind. 147, 109915. https://doi.org/10.1016/j.ecolind.2023.109915 (2023).
Bernery, C. et al. Freshwater fish invasions: A comprehensive review. Annu. Rev. Ecol. Evol. Syst. 53, 427–456. https://doi.org/10.1146/annurev-ecolsys-032522-015551 (2022).
Environmental Protection Bureau (DSPA). Survey of the Ecological Environment of Macao. (2020).
Shuai, F. et al. Nile tilapia (Oreochromis niloticus) invasions disrupt the functional patterns of fish community in a large subtropical river in China. Fish. Manag. Ecol. 26, 578–589. https://doi.org/10.1111/fme.12368 (2019).
Tsang, A. H. F. & Dudgeon, D. Do exotic poeciliids affect the distribution or trophic niche of native fishes? Absence of evidence from Hong Kong streams. Freshw. Biol. 66, 1751–1764. https://doi.org/10.1111/fwb.13789 (2021).
Tsang, A. H. F. & Dudgeon, D. Can the functional response to prey predict invasiveness? A comparison of native fishes and alien poeciliids in Hong Kong. Biol. Invasions. 23, 2143–2154. https://doi.org/10.1007/s10530-021-02493-9 (2021).
Tsang, A. H. F. & Dudgeon, D. A comparison of the ecological effects of two invasive poeciliids and two native fishes: a mesocosm approach. Biol. Invasions. 23, 1517–1532. https://doi.org/10.1007/s10530-020-02455-7 (2021).
Zhou, L., Wang, G., Kuang, T., Guo, D. & Li, G. Fish assemblage in the Pearl river estuary: Spatial-seasonal variation, environmental influence and trends over the past three decades. J. Appl. Ichthyol. 35, 884–895. https://doi.org/10.1111/jai.13912 (2019).
Qiao, L. et al. Spatial distribution and introduction pathways of non-native freshwater fish species in China. Water Biology Secur. 3, 100276. https://doi.org/10.1016/j.watbs.2024.100276 (2024).
Chan, J. et al. The non-native freshwater fishes of Hong kong: diversity, distributions, and origins. Raffles Bull. Zool. 71, 128–168. https://doi.org/10.26107/RBZ-2023-0012 (2023).
Yang, R., Cao, R., Gong, X. & Feng, J. Large shifts of niche and range in the golden Apple snail (Pomacea canaliculata), an aquatic invasive species. Ecosphere 14, e4391 (2023).
Xiao, R., Liu, D., Xu, Y., Li, T. & Ma, J. A review of the effects of limnoperna Fortunei (Dunker, 1857): invasion on hydraulic structures and ecosystems and their control. Sustainability 17, 2240 (2025).
Kennedy, V. S. The invasive dark falsemussel mytilopsis leucophaeata (Bivalvia: Dreissenidae): a literature review. Aquat. Ecol. 45, 163–183 (2011).
Roots, B. J. et al. Hypersalinity drives dramatic shifts in the invertebrate fauna of estuaries. Anim. (Basel). 15, 1629. https://doi.org/10.3390/ani15111629 (2025).
Peng, L. K. Characteristics of Water Quality in Macau Waters. (n.d.).
Ye, S. et al. Community characteristics and influencing factors of macrobenthos in Pearl river estuary. South. China Fisheries Sci. 19, 39–47. https://doi.org/10.12131/20230048 (2023).
Li, K. Z., Yin, J. Q., Huang, L. M. & Tan, Y. H. Spatial and Temporal variations of mesozooplankton in the Pearl river estuary, China. Estuar. Coast. Shelf Sci. 67, 543–552. https://doi.org/10.1016/j.ecss.2005.12.008 (2006).
Li, Z. et al. Seasonal changes in metacommunity assembly mechanisms of benthic macroinvertebrates in a subtropical river basin. Sci. Total Environ. 729, 139046. https://doi.org/10.1016/j.scitotenv.2020.139046 (2020).
Zhang, F. et al. Taxonomic and functional diversity of macroinvertebrate community along the elevation gradient and in different seasons in the upper Jinsha river (Qinghai-Tibet Plateau). Global Ecol. Conserv. 62, e03703. https://doi.org/10.1016/j.gecco.2025.e03703 (2025).
Lind, P. R., Robson, B. J. & Mitchell, B. D. The influence of reduced flow during a drought on patterns of variation in macroinvertebrate assemblages across a Spatial hierarchy in two lowland rivers. Freshw. Biol. 51, 2282–2295. https://doi.org/10.1111/j.1365-2427.2006.01650.x (2006).
Stitz, L., Fabbro, L. & Kinnear, S. Response of macroinvertebrate communities to seasonal hydrologic changes in three sub-tropical Australian streams. Environ. Monit. Assess. 189, 254–254. https://doi.org/10.1007/s10661-017-5957-8 (2017).
Attrill, M. J. & Thomas, R. M. Long-term distribution patterns of mobile estuarine invertebrates (Ctenophora, Cnidaria, crustacea: Decapoda) in relation to hydrological parameters. Mar. Ecol. Progress Ser. (Halstenbek). 143, 25–36. https://doi.org/10.3354/meps143025 (1996).
Abramova, E. & Tuschling, K. A 12-year study of the seasonal and interannual dynamics of mesozooplankton in the Laptev sea: significance of salinity regime and life cycle patterns. Glob. Planet Change. 48, 141–164. https://doi.org/10.1016/j.gloplacha.2004.12.010 (2005).
Uye, S., Shimazu, T., Yamamuro, M., Ishitobi, Y. & Kamiya, H. Geographical and seasonal variations in mesozooplankton abundance and biomass in relation to environmental parameters in lake Shinji–Ohashi River–Lake Nakaumi brackish-water system, Japan. J. Mar. Syst. 26, 193–207. https://doi.org/10.1016/S0924-7963(00)00054-3 (2000).
Steyaert, M. et al. Responses of intertidal nematodes to short-term anoxic events. J. Exp. Mar. Biol. Ecol. 345, 175–184. https://doi.org/10.1016/j.jembe.2007.03.001 (2007).
Fu, S., Cai, L., Cao, J. & Chen, X. Nematode responses to the invasion of exotic spartina in Mangrove wetlands in Southern China. Estuaries Coasts. 40, 1437–1449. https://doi.org/10.1007/s12237-017-0208-3 (2017).
Alves, A. S., Caetano, A., Costa, J. L., Costa, M. J. & Marques, J. C. Estuarine intertidal meiofauna and nematode communities as indicator of ecosystem’s recovery following mitigation measures. Ecol. Ind. 54, 184–196. https://doi.org/10.1016/j.ecolind.2015.02.013 (2015).
Lu, G., Pan, K., Zhu, A., Dong, Y. & Wang, W. X. Spatial-temporal variations and trends predication of trace metals in oysters from the Pearl river estuary of China during 2011–2018. Environ. Pollution (1987). 264, 114812. https://doi.org/10.1016/j.envpol.2020.114812 (2020).
Simaika, J. P. et al. Towards harmonized standards for freshwater biodiversity monitoring and biological assessment using benthic macroinvertebrates. Sci. Total Environ. 918, 170360. https://doi.org/10.1016/j.scitotenv.2024.170360 (2024).
Bogdan, K. G. & Gilbert, J. J. Seasonal patterns of feeding by natural populations of Keratella, Polyarthra, and bosmina: clearance rates, selectivities, and contributions to community grazing [Keratella cochlearis, Polyarthra vulgaris, Polyarthra dolichoptera, bosmina longirostris]. Limnol. Oceanogr. 27, 918–934. https://doi.org/10.4319/lo.1982.27.5.0918 (1982).
Botterell, Z. L., Lindeque, P. K., Thompson, R. C. & Beaumont, N. J. An assessment of the ecosystem services of marine zooplankton and the key threats to their provision. Ecosyst. Serv. 63, 101542 (2023).
Heininger, P., Höss, S., Claus, E., Pelzer, J. & Traunspurger, W. Nematode communities in contaminated river sediments. Environ. Pollut. 146, 64–76 (2007).
Majdi, N. & Traunspurger, W. Free-living nematodes in the freshwater food web: A review. J. Nematology. 47, 28 (2015).
Aston, R. J. Tubificids and water quality: A review. Environ. Pollut. 5, 1–10. https://doi.org/10.1016/0013-9327(73)90050-5 (1973).
Hong, A. H. et al. Examining molluscs as bioindicators of shrimp aquaculture effluent contamination in a Southeast Asian Mangrove. Ecol. Ind. 115, 106365 (2020).
Giarratano, E., Trovant, B. & Hernández-Moresino, R. D. Asian clam Corbicula fluminea as potential biomonitor of microplastics and metal(oid)s in a Patagonian river. Mar. Environ. Res. 198, 106548. https://doi.org/10.1016/j.marenvres.2024.106548 (2024).
Iacaruso, N. J., Reves, O. P., Merkelz, S. J., Waldrep, C. L. & Davis, M. A. A systematic review evaluating the performance of eDNA methods relative to conventional methods for biodiversity monitoring. Ecography (Copenhagen). https://doi.org/10.1002/ecog.07952 (2025).
Antich, A. et al. Marine biomonitoring with eDNA: can metabarcoding of water samples cut it as a tool for surveying benthic communities? Mol. Ecol. 30, 3175–3188. https://doi.org/10.1111/mec.15641 (2021).
Raupach, M. J. et al. The application of DNA barcodes for the identification of marine crustaceans from the North sea and adjacent regions. PloS One. 10, e0139421. https://doi.org/10.1371/journal.pone.0139421 (2015).
Atienza, S. et al. DNA metabarcoding of Deep-Sea sediment communities using COI: community Assessment, Spatio-Temporal patterns and comparison with 18S rDNA. Divers. (Basel). 12, 123. https://doi.org/10.3390/d12040123 (2020).
Hajibabaei, M., Porter, T. M., Wright, M. & Rudar, J. COI metabarcoding primer choice affects richness and recovery of indicator taxa in freshwater systems. PloS One. 14, e0220953. https://doi.org/10.1371/journal.pone.0220953 (2019).
Loos, L. M. & Nijland, R. Biases in bulk: DNA metabarcoding of marine communities and the methodology involved. Mol. Ecol. 30, 3270–3288. https://doi.org/10.1111/mec.15592 (2021).
dos Santos, R. A. & Blabolil, P. Comparison of bioinformatic pipelines for eDNA metabarcoding data analysis of fish populations. Fishes 10, 214. https://doi.org/10.3390/fishes10050214 (2025).
Alberdi, A., Aizpurua, O., Gilbert, M. T. P., Bohmann, K. & Mahon, A. Scrutinizing key steps for reliable metabarcoding of environmental samples. Methods Ecol. Evol. 9, 134–147. https://doi.org/10.1111/2041-210X.12849 (2018).
Chang, H., Ye, T., Xie, Z. & Liu, X. Application of environmental DNA in aquatic ecosystem monitoring: Opportunities, challenges and prospects. Water (Basel). 17, 661. https://doi.org/10.3390/w17050661 (2025).
ArcGIS. ArcGIS - System Software, < (2025). https://www.arcgis.com/index.html
Minamoto, T. et al. An illustrated manual for environmental DNA research: Water sampling guidelines and experimental protocols. Environ. DNA. 3, 8–13 (2021).
eDNA Methods Standardization Committee. Environmental DNA Sampling and Experiment Manual Version 2.1. (2019).
Leray, M. et al. A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: application for characterizing coral reef fish gut contents. Front. Zool. 10, 34 (2013).
Lobo, J. et al. Enhanced primers for amplification of DNA barcodes from a broad range of marine metazoans. BMC Ecol. 13, 34 (2013).
Buchner, D., Macher, T. H. & Leese, F. APSCALE: Advanced pipeline for simple yet comprehensive analyses of DNA metabarcoding data. Bioinformatics 38, 4817–4819 (2022).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584 (2016).
Leray, M., Knowlton, N. & Machida, R. J. MIDORI2: A collection of quality controlled, preformatted, and regularly updated reference databases for taxonomic assignment of eukaryotic mitochondrial sequences. Environ. Dna. 4, 894–907 (2022).
Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2012).
Iwasaki, W. et al. MitoFish and mitoannotator: a mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol. Biol. Evol. 30, 2531–2540 (2013).
Buchner, D. & Leese, F. BOLDigger–a python package to identify and organise sequences with the barcode of life data systems. Metabarcoding Metagenomics. 4, e53535 (2020).
Frøslev, T. G. et al. Algorithm for post-clustering curation of DNA amplicon data yields reliable biodiversity estimates. Nat. Commun. 8, 1188–1111. https://doi.org/10.1038/s41467-017-01312-x (2017).
Schnell, I. B., Bohmann, K. & Gilbert, M. T. P. Tag jumps illuminated – reducing sequence-to‐sample misidentifications in metabarcoding studies. Mol. Ecol. Resour. 15, 1289–1303. https://doi.org/10.1111/1755-0998.12402 (2015).
Callahan, B. J., McMurdie, P. J. & Holmes, S. P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 11, 2639–2643. https://doi.org/10.1038/ismej.2017.119 (2017).
Elbrecht, V., Peinert, B. & Leese, F. Sorting things out: assessing effects of unequal specimen biomass on DNA metabarcoding. Ecol. Evol. 7, 6918–6926. https://doi.org/10.1002/ece3.3192 (2017).
R. A Language and Environment for Statistical Computing v. v4.5.0 (Vienna, 2025).
GraphPad Prism version 9 for MacGraphPad Software, San Diego, California, USA, (2020).
Adobe Illustrator v. 29.8. San Jose, California, USA, (2025).
Anderson, M. J. & Walsh, D. C. I. PERMANOVA, ANOSIM, and the mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol. Monogr. 83, 557–574. https://doi.org/10.1890/12-2010.1 (2013).
Acknowledgements
We acknowledge members of the ME-BARCODE team from the University of Minho, particularly Tadeu Fontes, Jorge Moutinho, and André Ferreira, for their valuable ideas and support during the data analysis process as well as Darwi Htoo Saw and Steve Si Tou for the assistance during the field sampling.
Funding
This research was funded by the Drop by Drop Project through the WASH Foundation and Las Vegas Sands, grant number WASH/2023.
Author information
Authors and Affiliations
Contributions
M.K. Leong¹ prepared the Introduction and Methods sections, formatted the manuscript, conducted sampling and experiments, and assisted in figure production. I.H. Lau¹ was responsible for data collection and analysis, prepared the Results and Discussion sections, performed sampling and experiments, and produced figures. F.O. Costa² provided advice on the metabarcoding approach and data analysis techniques and contributed to reviewing the manuscript. M.P. Cabezas² offered guidance on metabarcoding data analysis methodologies and participated in reviewing and refining the manuscript. K. Tagulao¹ conceptualized the project, designed the sampling/experiments, secured funding, coordinated research activities, and contributed to reviewing and finalizing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Leong, M.K., Lau, I.H., Costa, F.O. et al. From fish to invertebrates: multi-marker eDNA metabarcoding for monitoring wetland biodiversity and non-indigenous species in Macao SAR China. Sci Rep (2026). https://doi.org/10.1038/s41598-026-39652-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-39652-8