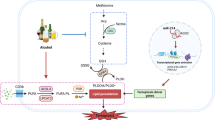
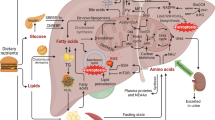
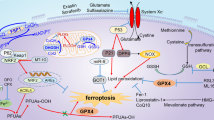
Abstract
While ferroptosis is implicated in alcoholic liver disease, its precise mechanisms of action are not fully defined. This study aims to investigate the protective role of the ferroptosis inhibitor Ferrostatin-1 (Fer-1) against alcoholic liver injury, exploring its underlying mechanisms. Using mice models of acute and chronic ethanol exposure, we assessed the effects of Fer-1. We evaluated liver function, histopathological damage, and key molecular markers related to ferroptosis, metabolism, and inflammation. Fer-1 significantly improved liver function and alleviated tissue damage, including lipid accumulation and fibrosis. It enhanced antioxidant capacity and reduced iron overload, oxidative stress, and lipid peroxidation. Furthermore, Fer-1 improved dysregulated iron and lipid metabolism and attenuated inflammation. Notably, these protective effects appear to be independent of the Nrf2/HO-1 pathway, autophagy, and NLRP3 inflammasome. Fer-1 exerts multi-faceted protection against alcoholic liver injury by specifically inhibiting ferroptosis. It blocks the vicious cycle of alcohol-induced metabolic imbalances, oxidative stress, and inflammation, offering new potential strategies for treating alcoholic liver disease.
Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Osna, N. A. et al. Alcohol-associated liver disease outcomes: Critical mechanisms of liver injury progression. Biomolecules 14. https://doi.org/10.3390/biom14040404 (2024).
Dasarathy, S. et al. Natural history and development of a novel composite endpoint in patients with alcohol-associated hepatitis: Data from a prospective multicenter study. Hepatology (Baltimore Md). https://doi.org/10.1097/hep.0000000000001513 (2025).
Ru, Q. et al. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal. Transduct. Target. Ther. 9. https://doi.org/10.1038/s41392-024-01969-z (2024).
Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590. https://doi.org/10.1038/s41586-021-03539-7 (2021).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282. https://doi.org/10.1038/s41580-020-00324-8 (2021).
Skouta, R. et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J. Am. Chem. Soc. 136, 4551–4556. https://doi.org/10.1021/ja411006a (2014).
Gong, Y. et al. AGER1 deficiency-triggered ferroptosis drives fibrosis progression in nonalcoholic steatohepatitis with type 2 diabetes mellitus. Cell. Death Discov. 9. https://doi.org/10.1038/s41420-023-01477-z (2023).
Liu, S., Chen, F., Han, J., Wang, L. & Dong, Y. Ferrostatin-1 improves neurological impairment induced by ischemia/reperfusion injury in the spinal cord through ERK1/2/SP1/GPX4. Exp. Neurol. 373, 114659. https://doi.org/10.1016/j.expneurol.2023.114659 (2024).
Luo, T. et al. Mechanistic insights into cadmium-induced nephrotoxicity: NRF2-driven HO-1 activation promotes ferroptosis via iron overload and oxidative stress in vitro. Free Radic. Biol. Med. 235, 162–175. https://doi.org/10.1016/j.freeradbiomed.2025.04.047 (2025).
Luo, C. et al. Autophagy induced by mechanical stress sensitizes cells to ferroptosis by NCOA4-FTH1 axis. Autophagy 21, 1263–1282. https://doi.org/10.1080/15548627.2025.2469129 (2025).
Wang, Y. et al. Dual-pathway targeted therapy for Parkinson’s disease: Biomimetic nanosomes inhibit ferroptosis and pyroptosis through NLRP3 inflammasome regulation. Bioactive Mater. 51, 825–840. https://doi.org/10.1016/j.bioactmat.2025.06.033 (2025).
Yu, L. et al. NLRP3 mediates NOXs-induced iron overload and inflammation but not oxidative damage in colons of DSS-treated mice. J. Inflamm. Res. 18, 4695–4708. https://doi.org/10.2147/jir.S509168 (2025).
Bertola, A., Mathews, S., Ki, S. H., Wang, H. & Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 8, 627–637. https://doi.org/10.1038/nprot.2013.032 (2013).
Luo, J. et al. Ferroptosis contributes to ethanol-induced hepatic cell death via labile iron accumulation and GPx4 inactivation. Cell. Death Discov. 9. https://doi.org/10.1038/s41420-023-01608-6 (2023).
Wang, H. et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology (Baltimore Md) 66, 449–465. https://doi.org/10.1002/hep.29117 (2017).
Yuan, X. et al. Heme oxygenase 1 alleviates nonalcoholic steatohepatitis by suppressing hepatic ferroptosis. Lipids Health Dis. 22. https://doi.org/10.1186/s12944-023-01855-7 (2023).
Jia, M. et al. Ferroptosis as a new therapeutic opportunity for nonviral liver disease. Eur. J. Pharmacol. 908. https://doi.org/10.1016/j.ejphar.2021.174319 (2021).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698. https://doi.org/10.1038/s41586-019-1707-0 (2019).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692. https://doi.org/10.1038/s41586-019-1705-2 (2019).
Liu, B. et al. SFTSV induces liver ferroptosis through m6A-related ferritinophagy. Autophagy 21, 2353–2366. https://doi.org/10.1080/15548627.2025.2503564 (2025).
Chen, Y., Gao, W., Cai, K., Yang, L. & Chu, H. Ferroptosis and gut microbiota: A new horizon in alcohol-associated liver disease management. Cell. Mol. Life Sci. 82, 282. https://doi.org/10.1007/s00018-025-05815-5 (2025).
Seitz, H. K. et al. Alcoholic liver disease. Nat. Rev. Dis. Prim. 4. https://doi.org/10.1038/s41572-018-0014-7 (2018).
Li, L. X., Guo, F. F., Liu, H. & Zeng, T. Iron overload in alcoholic liver disease: Underlying mechanisms, detrimental effects, and potential therapeutic targets. Cell. Mol. Life Sci. 79, 201. https://doi.org/10.1007/s00018-022-04239-9 (2022).
Silva, I., Rausch, V., Seitz, H. K. & Mueller, S. Does hypoxia cause carcinogenic iron accumulation in alcoholic liver disease (ALD)? Cancers 9. https://doi.org/10.3390/cancers9110145 (2017).
Galy, B., Conrad, M. & Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 25, 133–155. https://doi.org/10.1038/s41580-023-00648-1 (2024).
Xiao, X. et al. Regulation of iron homeostasis by hepatocyte TfR1 requires HFE and contributes to hepcidin suppression in β-thalassemia. Blood 141, 422–432. https://doi.org/10.1182/blood.2022017811 (2023).
Xu, Y., Li, Y., Li, J. & Chen, W. Ethyl carbamate triggers ferroptosis in liver through inhibiting GSH synthesis and suppressing Nrf2 activation. Redox Biol. 53, 102349. https://doi.org/10.1016/j.redox.2022.102349 (2022).
Zheng, C. et al. Evidence for alcohol-mediated hemolysis and erythrophagocytosis. Redox Biol. 85, 103742. https://doi.org/10.1016/j.redox.2025.103742 (2025).
Chen, C. et al. H(2)O(2)-mediated autophagy during ethanol metabolism. Redox Biol. 46, 102081. https://doi.org/10.1016/j.redox.2021.102081 (2021).
Hou, W. et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425–1428. https://doi.org/10.1080/15548627.2016.1187366 (2016).
de Carvalho Ribeiro, M. & Szabo, G. Role of the inflammasome in liver disease. Annu. Rev. Pathol. 17, 345–365. https://doi.org/10.1146/annurev-pathmechdis-032521-102529 (2022).
Gao, H. et al. Cell-to-cell and organ-to-organ crosstalk in the pathogenesis of alcohol-associated liver disease. eGastroenterology 2. https://doi.org/10.1136/egastro-2024-100104 (2024).
Anandhan, A., Dodson, M., Schmidlin, C. J., Liu, P. & Zhang, D. D. Breakdown of an ironclad defense system: The critical role of NRF2 in mediating ferroptosis. Cell. Chem. Biol. 27, 436–447. https://doi.org/10.1016/j.chembiol.2020.03.011 (2020).
Yuan, C. et al. Curcumin induces ferroptosis and apoptosis in osteosarcoma cells by regulating Nrf2/GPX4 signaling pathway. Experimental Biology Med. (Maywood N J). 248, 2183–2197. https://doi.org/10.1177/15353702231220670 (2023).
Shi, W. et al. Intracellular iron deficiency and abnormal metabolism, not ferroptosis, contributes to homocysteine-induced vascular endothelial cell death. Biomedicines 12. https://doi.org/10.3390/biomedicines12102301 (2024).
Jiang, H. et al. Ferrostatin-1 ameliorates liver dysfunction via reducing iron in thioacetamide-induced acute liver injury in mice. Front. Pharmacol. 13, 869794. https://doi.org/10.3389/fphar.2022.869794 (2022).
Sui, Y. et al. Targeting the regulation of iron homeostasis as a potential therapeutic strategy for nonalcoholic fatty liver disease. Metab. Clin. Exp. 157, 155953. https://doi.org/10.1016/j.metabol.2024.155953 (2024).
Subramaiyam, N. Insights of mitochondrial involvement in alcoholic fatty liver disease. J. Cell. Physiol. 238, 2175–2190. https://doi.org/10.1002/jcp.31100 (2023).
Yang, C. et al. Loss of GCN5L1 exacerbates damage in alcoholic liver disease through ferroptosis activation. Liver International: Official J. Int. Association Study Liver. 44, 1924–1936. https://doi.org/10.1111/liv.15936 (2024).
Ma, W., Wang, Y. & Liu, J. NLRP3 inflammasome activation in liver disorders: From molecular pathways to therapeutic strategies. J. Inflamm. Res. 18, 8277–8294. https://doi.org/10.2147/jir.S532908 (2025).
Acknowledgements
This work was supported by Yunnan Provincial Clinical Medicine Center for Digestive System Diseases (2024YNLCYXZX0111), and Yunnan Fundamental Research Projects (202301AU070167). Yunnan Labreal Biotechnology Co.,Ltd provided the research materials for the animal experiment. Although the animal experiment was performed on a private company’s platform, the company did not participate in writing, reviewing, and publishing this manuscript. No economic interests were revealed. We are grateful to Hui Li and the experimental animal team of the Yunnan Labreal Biotechnology Co.,Ltd for providing technical support in constructing the mice model for this work.
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.L.; methodology, Y.L. and S.Z.; Investigation, Z.H., W.Y., X.L., Z.X., and L.Y.; Software Z.H., W.Y., and Z.A.; Writing—original draft, Y.L. and Z.H.; Writing—review & editing, S.Z.; Funding acquisition, Y.L.; Supervision, S.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, L., Zhang, H., Wang, Y. et al. Ferrostatin 1 exerts multifaceted hepatic protection against alcoholic liver injury by inhibiting ferroptosis. Sci Rep (2026). https://doi.org/10.1038/s41598-026-39849-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-39849-x