Abstract
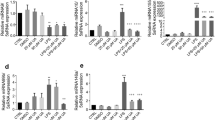
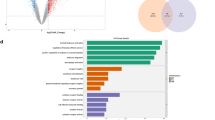
We investigated the protective effects of skipjack tuna (Katsuwonus pelamis) bone-derived biocalcium (Bio) against dexamethasone-induced atrophy in C2C12 myotubes. Bio rescued atrophic morphology, increasing myotube diameter dose-dependently. It mitigated inflammation by suppressing nitric oxide production and the expression and concentration of proinflammatory cytokines (IL-6, TNF-α, IL-1β) significantly and dose-dependently. Bio restored protein turnover balance by downregulating MuRF1 and atrogin-1 while upregulating MTOR. At 5–20 µg/mL, Bio downregulated total NF-κB p65, p38 mitogen-activated protein kinase (MAPK), and FoxO3a and upregulated Akt expression. Crucially, Bio dose-dependently downregulated primary-, precursor-, and mature-microRNA-29b. In Bio-treated, dexamethasone-treated C2C12 myotubes, microRNA-29b inhibitor co-transfection significantly increased myogenin and MyoD expression, whereas microRNA-29b mimic co-transfection suppressed these myogenic markers, confirming the inhibitory role of microRNA-29b. Molecular docking simulations confirmed strong binding affinities between microRNA-29b and myogenin/MyoD. These results demonstrate that Bio exerts anti-atrophy effects by disrupting the microRNA-29b-mediated block on myogenesis and modulating inflammatory responses, protein turnover, and key signaling pathways. Collectively, skipjack tuna-derived Bio shows promise as a functional food supplement for sarcopenia prevention and management.
Similar content being viewed by others
Data availability
The data generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Mankhong, S. et al. Experimental models of sarcopenia: bridging molecular mechanism and therapeutic strategy. Cells 9, 1385. https://doi.org/10.3390/cells9061385 (2020).
Wang, T., Zhou, D. & Hong, Z. Sarcopenia and cachexia: molecular mechanisms and therapeutic interventions. MedComm 6, e70030. https://doi.org/10.1002/mco2.70030 (2025).
Ganapathy, A. & Nieves, J. W. Nutrition and sarcopenia—What do we know? Nutrients 12, 1755. https://doi.org/10.3390/nu12061755 (2020).
Liu, S., Zhang, L. & Li, S. Advances in nutritional supplementation for sarcopenia management. Front. Nutr. 10, 1189522. https://doi.org/10.3389/fnut.2023.1189522 (2023).
Bhattacharya, S., Bhadra, R., Schols, A. M. W. J., van Helvoort, A. & Sambashivaiah, S. Nutrition in the prevention and management of sarcopenia - A special focus on Asian Indians. Osteoporos. Sarcopenia. 8, 135–144 (2022).
Benjakul, S., Mad-Ali, S., Senphan, T. & Sookchoo, P. Characteristics of biocalcium from pre-cooked skipjack tuna bone as affected by different treatments. Waste Biomass Valorization. 9, 1369–1377 (2018).
Saetang, J. et al. Bio-calcium from skipjack tuna frame attenuates bone loss in ovariectomy-induced osteoporosis rats. Mar. Drugs. 22, 472. https://doi.org/10.3390/md22100472 (2024).
Benjakul, S., Mad-Ali, S., Senphan, T. & Sookchoo, P. Biocalcium powder from precooked skipjack tuna bone: production and its characteristics. J. Food Biochem. 41, e12412. https://doi.org/10.1111/jfbc.12412 (2017).
Li, J. et al. miR-29b contributes to multiple types of muscle atrophy. Nat. Commun. 8, 15201. https://doi.org/10.1038/ncomms15201 (2017).
Hu, Z. et al. MicroRNA-29 induces cellular senescence in aging muscle through multiple signaling pathways. Aging (Albany NY). 6, 160–175 (2014).
Li, J. et al. Angiotensin II-induced muscle atrophy via PPARγ suppression is mediated by miR-29b. Mol. Ther. Nucleic Acids. 23, 743–756 (2021).
Wei, W. et al. miR-29 targets Akt3 to reduce proliferation and facilitate differentiation of myoblasts in skeletal muscle development. Cell. Death Dis. 4, e668. https://doi.org/10.1038/cddis.2013.184 (2013).
Wang, H. et al. NF-κB–YY1–miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 14, 369–381 (2008).
Zhou, L. et al. A novel target of microRNA-29, ring1 and YY1-binding protein (Rybp), negatively regulates skeletal myogenesis. J. Biol. Chem. 287, 25255–25265 (2012).
Li, J. et al. Engineered circular RNA circmiR-29b attenuates muscle atrophy by sponging miR-29b. Adv. Th. 5, 2200029. https://doi.org/10.1002/adtp.202200029 (2022).
Tang, W. et al. MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br. J. Cancer. 110, 450–458 (2014).
Wang, X. S. et al. MicroRNA-29a and microRNA-142-3p are regulators of myeloid differentiation and acute myeloid leukemia. Blood 119, 4992–5004 (2012).
Gao, Y., Arfat, Y., Wang, H. & Goswami, N. Muscle atrophy induced by mechanical unloading: mechanisms and potential countermeasures. Front. Physiol. 9, 235. https://doi.org/10.3389/fphys.2018.00235 (2018).
Permpoon, U., Moon, J., Kim, C. Y. & Nam, T.-g. Glucocorticoid-mediated skeletal muscle atrophy: molecular mechanisms and potential therapeutic targets. Int. J. Mol. Sci. 26, 7616. https://doi.org/10.3390/ijms26157616 (2025).
Wang, Y. et al. Nutraceuticals in the prevention and treatment of the muscle atrophy. Nutrients 13 (1914). https://doi.org/10.3390/nu13061914 (2021).
Rahman, M. M. et al. Muscle-protective effect of carnosine against dexamethasone-induced muscle atrophy in C2C12 myotube. J. Nutr. Sci. Vitaminol. 70, 219–227 (2024).
Canovas, B. & Nebreda, A. R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell. Biol. 22, 346–366 (2021).
Shin, H. E. et al. MicroRNAs as commonly expressed biomarkers for sarcopenia and frailty: A systematic review. Exp. Gerontol. 197, 112600. https://doi.org/10.1016/j.exger.2024.112600 (2024).
He, N. et al. Circulating miR-29b decrease in response to sarcopenia in patients with cardiovascular risk factors in older Chinese. Front. Cardiovasc. Med. 9, 1094388. https://doi.org/10.3389/fcvm.2022.1094388 (2022).
Braile, M. et al. Role of MicroRNAs in osteosarcopenic obesity/adiposity: a scoping review. Cells 14, 802. https://doi.org/10.3390/cells14110802 (2025).
Li, J. et al. CRISPR/Cas9-mediated miR-29b editing as a treatment of different types of muscle atrophy in mice. Mol. Ther. 28, 1359–1372 (2020).
Dakhovnik, A. et al. A collagen amino acid composition supplementation reduces biological age in humans and increases health and lifespan in vivo. Npj Aging. 11, 91. https://doi.org/10.1038/s41514-025-00280-7 (2025).
Jiang, J., Chen, G., Li, Y., Zhao, Q. & Chen, Z. Serum calcium levels and the risk of sarcopenia in young adults: insights from NHANES 2011–2018. Front. Nutr. 12, 1526879. https://doi.org/10.3389/fnut.2025.1526879 (2025).
Kheeree, N. et al. Discovery of calcium-binding peptides derived from defatted lemon basil seeds with enhanced calcium uptake in human intestinal epithelial cells, Caco-2. Sci. Rep. 12, 4659. https://doi.org/10.1038/s41598-022-08380-0 (2022).
Xie, W. Q. et al. Mouse models of sarcopenia: classification and evaluation. J. Cachexia Sarcopenia Muscle. 12, 538–554. https://doi.org/10.1002/jcsm.12709 (2021).
Li, P. W. C. et al. Resistance-based exercise intervention for patients with coronary artery disease and sarcopenia: a pilot randomized controlled trial. Eur. J. Cardiovasc. Nurs. 24, 736–745. https://doi.org/10.1093/eurjcn/zvaf041 (2025).
Morgan, G. S. et al. A pilot randomised controlled trial of physical activity facilitation for older adults: feasibility study findings. Pilot Feasibility Stud. 5, 40. https://doi.org/10.1186/s40814-019-0414-9 (2019).
Sinclair, B. et al. Pilot randomised controlled trial of a novel form of exercise on parkinsonism symptoms. BMJ Neurol. Open. 8, e001248. https://doi.org/10.1136/bmjno-2025-001248 (2026).
Zhou, H. et al. Impacts of resistance training combined with vibration training on the IGF-1/PI3K/AKT/FOXO3 axis and clinical outcomes in patients with sarcopenia: A protocol for a randomized controlled trial. PLOS One. 20, e0333343. https://doi.org/10.1371/journal.pone.0333343 (2025).
Wang, B. Y. H. et al. Mesenchymal stem cells alleviate dexamethasone-induced muscle atrophy in mice and the involvement of ERK1/2 signalling pathway. Stem Cell. Res. Ther. 14, 195. https://doi.org/10.1186/s13287-023-03418-0 (2023).
Nakagawara, K., Takeuchi, C. & Ishige, K. 5′-CMP and 5′-UMP alleviate dexamethasone-induced muscular atrophy in C2C12 myotubes. Biochem. Biophys. Rep. 34, 101460. https://doi.org/10.1016/j.bbrep.2023.101460 (2023).
Sani, A. et al. Inhibitory effects of curcuminoids on dexamethasone-induced muscle atrophy in differentiation of C2C12 cells. Phytomedicine Plus. 1, 100012. https://doi.org/10.1016/j.phyplu.2020.100012 (2021).
Jantarawong, S. et al. CRE-Ter enhances murine bone differentiation, improves muscle cell atrophy, and increases Irisin expression. PLOS One. 20, e0338571. https://doi.org/10.1371/journal.pone.0338571 (2025).
Cheng, M. et al. MicroRNA-181a suppresses parkin-mediated mitophagy and sensitizes neuroblastoma cells to mitochondrial uncoupler-induced apoptosis. Oncotarget 7, 42274. https://doi.org/10.18632/oncotarget.9786 (2016).
Madhyastha, R. et al. The pivotal role of microRNA-21 in osteoclastogenesis Inhibition by anthracycline glycoside aloin. J. Nat. Med. 73, 59–66 (2019).
Liu, Z. et al. miR-29a-3p compositely regulates the COL6A6/PTEN-PI3K/Akt/CUX1 feedback loop to participate in the proliferation and invasion of pituitary adenomas. J. Mol. Histol. 56, 172. https://doi.org/10.1007/s10735-025-10436-0 (2025).
Zhao, W. et al. Novel miR-29b target regulation patterns are revealed in two different cell lines. Sci. Rep. 9 https://doi.org/10.1038/s41598-019-53868-x (2019).
Jantarawong, S., Wathanaphanit, P., Panichayupakaranant, P. & Pengjam, Y. Prediction of ADMET profile and anti-inflammatory potential of chamuangone. Sci. Rep. 15, 2963. https://doi.org/10.1038/s41598-025-86809-y (2025).
Jantarawong, S. et al. Binary curcuminoid complex CRE-Bin inhibits osteoclast differentiation by suppressing the canonical NF-κB signaling pathway and modulating microRNA-223 expression. Sci. Rep. 15, 21255. https://doi.org/10.1038/s41598-025-04314-8 (2025).
Zhou, Y. et al. miR-223 overexpression inhibits doxorubicin-induced autophagy by targeting FOXO3a and reverses chemoresistance in hepatocellular carcinoma cells. Cell. Death Dis. 10, 843. https://doi.org/10.1038/s41419-019-2053-8 (2019).
Consortium, T. U. UniProt: The universal protein knowledgebase in 2025. Nucleic Acids Res. 53, D609–D617 (2024).
Burley, S. K. et al. Updated resources for exploring experimentally-determined PDB structures and computed structure models at the RCSB protein data bank. Nucleic Acids Res. 53, D564–D574 (2024).
Kozomara, A., Birgaoanu, M. & Griffiths-Jones, S. miRBase: From MicroRNA sequences to function. Nucleic Acids Res. 47, D155–D162 (2018).
Biesiada, M., Purzycka, K. J., Szachniuk, M., Blazewicz, J. & Adamiak, R. W. Automated RNA 3D structure prediction with RNAComposer. Methods Mol. Biol. 1490, 199–215 (2016).
Yan, Y., Zhang, D., Zhou, P., Li, B. & Huang, S. Y. HDOCK: A web server for protein–protein and protein–DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 45, W365–W373 (2017).
Acknowledgements
This research was supported by the National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant No. MET6901024S).
Funding
This research was supported by the National Science, Research and Innovation Fund (NSRF) and Prince of Songkla University (Grant No. MET6901024S).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.P.; methodology, S.J., T.S., C.Y., N.T., P.W., P.S., Y.Y., K.H., and Y.P.; validation, S.J., and Y.P.; formal analysis, S.J., C.Y., N.T., and Y.P.; investigation, S.J., T.S., C.Y., N.T., P.W., P.S., Y.Y., K.H., and Y.P.; resources, Y.P.; writing—original draft preparation, S.J.; writing—review and editing, S.J. and Y.P.; visualization, S.J., and Y.P.; supervision, Y.P.; project administration, Y.P.; funding acquisition, Y.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jantarawong, S., Senphan, T., Youngruk, C. et al. Skipjack tuna bone derived biocalcium ameliorates C2C12 myotube atrophy through microRNA29b regulation. Sci Rep (2026). https://doi.org/10.1038/s41598-026-39977-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-39977-4