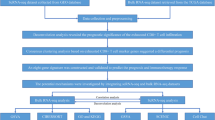
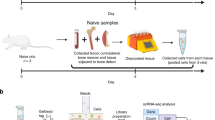
Abstract
Traumatic brain injury (TBI) can alter various immune functions, including immunosuppression, and constitutes a risk factor for nosocomial infections and organ dysfunction. Although TBI can induce a decline in immune cell function, the detailed mechanisms remains to be elucidated. We aim to explore transcriptional signatures associated with post-TBI immune alterations in a pilot cohort using a comprehensive transcriptome analysis of immune cells. Three patients with traumatic brain injury and acute subdural hematoma were admitted to our hospital. We focused on three major subsets of immune cells responsible for the immune response: CD4 + T cells, CD8 + T cells, and monocytes. We evaluated the changes in immune function after injury using comprehensive transcriptome analysis. Blood samples were collected immediately after admission and one week later, and the data were compared with those of healthy volunteers. CD4 + , CD8 + T cells, and monocytes, the expression of pathways involved in cellular metabolism—including oxidative phosphorylation, mTORC1 signaling, MYC targets V1 and V2, and the unfolded protein response—was downregulated on day 7 compared with day 1. These findings suggest a transition from marked immune activation and metabolic upregulation on day 1 to an attenuated immune response by day 7. In CD4 + T cells, pathways associated with tissue remodeling and repair, such as hedgehog signaling and epithelial–mesenchymal transition, were upregulated from days 1 to 7, indicating a shift from inflammatory responses toward inflammation resolution and tissue repair. In this pilot study, comprehensive transcriptome profiling suggests time-dependent transcriptional shifts in CD4⁺ T cells, CD8⁺ T cells, and monocytes after TBI. These findings should be interpreted as hypothesis-generating and provide a framework for larger, confirmatory studies.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article. The raw data from this study were submitted to the Gene Expression Omnibus under accession numbers GSE285385 and GSE314947.
Abbreviations
- AIS-98:
-
Abbreviated injury scale 98
- CARS:
-
Compensatory anti-inflammatory response syndrome
- GCS:
-
Glasgow coma scale
- GOS:
-
Glasgow outcome scale
- HLA:
-
Human leukocyte antigen
- IQR:
-
Interquartile range
- ISS:
-
Injury severity score
- PBMC:
-
Peripheral blood mononuclear cell
- SIRS:
-
Systemic inflammatory response syndrome
- TBI:
-
Traumatic brain injury
References
Taylor, C. A., Bell, J. M., Breiding, M. J. & Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ. 66(9), 1–16 (2017).
Wirtz, M. R. et al. Autonomic nervous system activity and the risk of nosocomial infection in critically ill patients with brain injury. Intensive Care Med Exp. 8(1), 69 (2020).
Li, Y., Liu, C., Xiao, W., Song, T. & Wang, S. Incidence, Risk Factors, and Outcomes of Ventilator-Associated Pneumonia in Traumatic Brain Injury: A Meta-analysis. Neurocrit Care. 32(1), 272–285 (2020).
Bouras, M., Asehnoune, K. & Roquilly, A. Immune modulation after traumatic brain injury. Front Med (Lausanne). 9, 995044 (2022).
Yates, A. G., Anthony, D. C., Ruitenberg, M. J. & Couch, Y. Systemic immune response to traumatic cns injuries-are extracellular vesicles the missing link?. Front Immunol. 10, 2723 (2019).
Soliman, A. M. & Barreda, D. R. Acute inflammation in tissue healing. Int J Mol Sci. 24(1), 641 (2022).
Matzinger, P. The danger model: a renewed sense of self. Science 296(5566), 301–305 (2002).
Caceres, E., Olivella, J. C., Di Napoli, M., Raihane, A. S. & Divani, A. A. Immune Response in Traumatic Brain Injury. Curr Neurol Neurosci Rep. 24(12), 593–609 (2024).
Bao, W., Lin, Y. & Chen, Z. The Peripheral Immune System and Traumatic Brain Injury: Insight into the role of T-helper cells. Int J Med Sci. 18(16), 3644–3651 (2021).
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. 1992 Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 101(6): 1644–55.
Adib-Conquy, M. & Cavaillon, J. M. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 101(1), 36–47 (2009).
Bone, R. C. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann Intern Med. 125(8), 680–687 (1996).
Bortolotti, P., Faure, E. & Kipnis, E. Inflammasomes in tissue damages and immune disorders after trauma. Front Immunol. 9, 1900 (2018).
Yang, Y. W. et al. Dynamics of immune responses are inconsistent when trauma patients are grouped by injury severity score and clinical outcomes. Sci Rep. 13(1), 1391 (2023).
Murphy, T., Paterson, H., Rogers, S., Mannick, J. A. & Lederer, J. A. Use of intracellular cytokine staining and bacterial superantigen to document suppression of the adaptive immune system in injured patients. Ann Surg. 238(3), 401–410 (2003).
Hua, R. et al. Decreased levels of perforin-positive lymphocytes are associated with posttraumatic complications in patients with major trauma. Injury 45(12), 2089–2095 (2014).
Ni Choileain, N. et al. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 176(1), 225–236 (2006).
Vester, H., Dargatz, P., Huber-Wagner, S., Biberthaler, P. & van Griensven, M. HLA-DR expression on monocytes is decreased in polytraumatized patients. Eur J Med Res. 20, 84 (2015).
Livingston, D. H., Appel, S. H., Wellhausen, S. R., Sonnenfeld, G. & Polk, H. C. Jr. Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch Surg. 123(11), 1309–1312 (1988).
Matsumoto, H., Ogura, H. & Oda, J. Analysis of comprehensive biomolecules in critically ill patients via bioinformatics technologies. Acute Med Surg. 11(1), e944 (2024).
Xiao, W. et al. A genomic storm in critically injured humans. J Exp Med. 208(13), 2581–2590 (2011).
Chen, T. et al. The independent prognostic value of global epigenetic alterations: An analysis of single-cell ATAC-seq of circulating leukocytes from trauma patients followed by validation in whole blood leukocyte transcriptomes across three etiologies of critical illness. EBioMedicine 76, 103860 (2022).
Karibe, H. et al. Surgical management of traumatic acute subdural hematoma in adults: a review. Neurol Med Chir (Tokyo). 54(11), 887–894 (2014).
American Thoracic, S. Infectious Diseases Society of A. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 171(4), 388–416 (2005).
Yamaguchi Y, Kato Y, Edahiro R, Sondergaard JN, Murakami T, Amiya S, et al. Consecutive BNT162b2 mRNA vaccination induces short-term epigenetic memory in innate immune cells. JCI Insight. 2022;7(22).
Chen, Y., Lun, A. T. & Smyth, G. K. From reads to genes to pathways differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. Research 5, 1438 (2016).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1(6), 417–425 (2015).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 102(43), 15545–15550 (2005).
Chang, J. C. et al. Soluble adenylyl cyclase regulates the cytosolic NADH/NAD(+) redox state and the bioenergetic switch between glycolysis and oxidative phosphorylation. Biochim Biophys Acta Bioenerg. 1862(4), 148367 (2021).
Shyer, J. A., Flavell, R. A. & Bailis, W. Metabolic signaling in T cells. Cell Res. 30(8), 649–659 (2020).
Waickman, A. T. & Powell, J. D. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol Rev. 249(1), 43–58 (2012).
Marchingo JM, Sinclair LV, Howden AJ, Cantrell DA. 2020 Quantitative analysis of how Myc controls T cell proteomes and metabolic pathways during T cell activation. Elife.9.
Ren, B. et al. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 16(2), 245–256 (2002).
Nair, K. A. 2nd. & Liu, B. Navigating the landscape of the unfolded protein response in CD8(+) T cells. Front Immunol. 15, 1427859 (2024).
Hu, Q. et al. Resting T cells are hypersensitive to DNA damage due to defective DNA repair pathway. Cell Death Dis. 9(6), 662 (2018).
Viasus, D. et al. Whole-blood gene expression profiles associated with mortality in community-acquired pneumonia. Biomedicines. 11(2), 429 (2023).
Hirschberger, S. et al. Mitochondrial damage drives T-cell immunometabolic paralysis after major surgery. EMBO Mol Med. 17(12), 3329–3354 (2025).
Martin, M. D., Badovinac, V. P. & Griffith, T. S. CD4 T Cell Responses and the Sepsis-Induced Immunoparalysis State. Front Immunol. 11, 1364 (2020).
Shen, X., Peng, Y. & Li, H. The Injury-Related Activation of Hedgehog Signaling Pathway Modulates the Repair-Associated Inflammation in Liver Fibrosis. Front Immunol. 8, 1450 (2017).
Youssef, K. K. et al. Two distinct epithelial-to-mesenchymal transition programs control invasion and inflammation in segregated tumor cell populations. Nat Cancer. 5(11), 1660–1680 (2024).
Topchyan, P., Lin, S. & Cui, W. The Role of CD4 T Cell Help in CD8 T Cell Differentiation and Function During Chronic Infection and Cancer. Immune Netw. 23(5), e41 (2023).
Montauti, E., Oh, D. Y. & Fong, L. CD4(+) T cells in antitumor immunity. Trends Cancer. 10(10), 969–985 (2024).
Pattu, V., Krause, E., Chang, H. F., Rettig, J. & Li, X. IFNgamma Expression Correlates with Enhanced Cytotoxicity in CD8+ T Cells. Int J Mol Sci. 26(14), 7024 (2025).
Jensen, I. J., Sjaastad, F. V., Griffith, T. S. & Badovinac, V. P. Sepsis-induced T Cell immunoparalysis: the ins and outs of impaired T Cell immunity. J Immunol. 200(5), 1543–1553 (2018).
Han, Z. et al. The role of monocytes in thrombotic diseases: a review. Front Cardiovasc Med. 10, 1113827 (2023).
Weber, B. et al. Diagnostic and prognostic potential of exosomal cytokines IL-6 and IL-10 in polytrauma patients. Int J Mol Sci. 24(14), 11830 (2023).
Morrow, K. N., Coopersmith, C. M. & Ford, M. L. IL-17, IL-27, and IL-33: A novel axis linked to immunological dysfunction during sepsis. Front Immunol. 10, 1982 (2019).
Acknowledgements
We thank the Department of Medical Informatics, Osaka University Hospital, Osaka, Japan, for their cooperation in the collection of data from medical records. We also thank the medical staff who participated in this study.
Funding
This study was supported by the General Insurance Association of Japan (grant to HI 2021), a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (24K12153) to HI, and a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (23K27701) to HO.
Author information
Authors and Affiliations
Contributions
HI, MI, HM, HO, and DO were involved in the conception and design of the study. HI performed the literature review. HI and MI acquired the data and performed the analysis and interpretation. HI drafted the manuscript. HI, MI, HM, HO, and DO were involved in the critical revision of the manuscript. All authors contributed to the discussions, managed the study, and read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board of the University of Osaka hospital (Approval Number: 885) and complied with the principles of the Declaration of Helsinki. Written informed consent for the collection of blood samples and the use of clinical data was obtained from the patients or their relatives, and from healthy volunteers.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ito, H., Ishikawa, M., Matsumoto, H. et al. Gene expression changes in lymphocytes and monocytes from patients with traumatic brain injury. Sci Rep (2026). https://doi.org/10.1038/s41598-026-39991-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-39991-6