Abstract
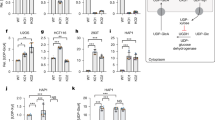
The mechanisms underlying cellular damage induced by the mitochondrially encoded NADH dehydrogenase subunit 4 (MT-ND4) with R340H mutation caused by the variant m.11778G > A in complex I are intricate. Numerous studies indicate that mitochondria play a primary role in cellular death due to this mutation. However, the detailed pathological effects remain incompletely elucidated. To decipher the specific impacts of this mutation on cellular death, mitochondrial dysfunction was investigated in 661 W cells expressing exogenous Mut-ND4 (m.G11778A). Importantly, the oxygen consumption rate (OCR) assessed by Seahorse XF analyzer exhibited a significant decrease under galactose conditions and an excessive production of reactive oxygen species (ROS). Conversely, the activity levels of catalase (CAT), superoxide dismutase (SOD), and glutathione disulfide (GSSG) were decreased, leading to increased cell death in cells expressing Mut-ND4 (m.G11778A) under galactose conditions. In addition, structural disruptions in the optic nerves of mice subjected to Mut-ND4-AAV infection were revealed. These findings suggest that Mut-ND4 (m.G11778A) contributes to cellular injury and an oxidative stress imbalance, characterized by decreased mitochondrial oxygen consumption, increased oxidative products, and reduced antioxidant capacity.
Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Wallace, D. C. et al. Mitochondrial DNA mutation associated with leber’s hereditary optic neuropathy. Science 242, 1427–1430. https://doi.org/10.1126/science.3201231 (1988).
Jurkute, N. & Yu-Wai-Man, P. Leber hereditary optic neuropathy: bridging the translational gap. Curr. Opin. Ophthalmol. 28, 403–409. https://doi.org/10.1097/icu.0000000000000410 (2017).
Carelli, V. et al. Biochemical features of MtDNA 14484 (ND6/M64V) point mutation associated with leber’s hereditary optic neuropathy. Ann. Neurol. 45, 320–328 (1999).
Qi, X., Sun, L., Lewin, A. S., Hauswirth, W. W. & Guy, J. The mutant human ND4 subunit of complex I induces optic neuropathy in the mouse. Invest. Ophthalmol. Vis. Sci. 48, 1–10. https://doi.org/10.1167/iovs.06-0789 (2007).
Koilkonda, R. D. & Guy, J. Leber’s hereditary optic neuropathy-gene therapy: from benchtop to bedside. J. Ophthalmol. 2011 179412. https://doi.org/10.1155/2011/179412 (2011).
Chen, B. S., Yu-Wai-Man, P. & Newman, N. J. Developments in the treatment of leber hereditary optic neuropathy. Curr. Neurol. Neurosci. Rep. 22, 881–892. https://doi.org/10.1007/s11910-022-01246-y (2022).
Davila-Siliezar, P., Carter, M., Milea, D. & Lee, A. G. Leber hereditary optic neuropathy: new and emerging therapies. Curr. Opin. Ophthalmol. 33, 574–578. https://doi.org/10.1097/icu.0000000000000891 (2022).
Sobh, M. et al. Safety and efficacy of Adeno-Associated viral gene therapy in patients with retinal degeneration: A systematic review and Meta-Analysis. Transl Vis. Sci. Technol. 12, 24. https://doi.org/10.1167/tvst.12.11.24 (2023).
Tan, E. et al. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in Transgenic mice. Invest. Ophthalmol. Vis. Sci. 45, 764–768. https://doi.org/10.1167/iovs.03-1114 (2004).
Sayyad, Z., Sirohi, K., Radha, V. & Swarup, G. 661 W is a retinal ganglion precursor-like cell line in which glaucoma-associated optineurin mutants induce cell death selectively. Sci. Rep. 7, 16855. https://doi.org/10.1038/s41598-017-17241-0 (2017).
Guy, J. et al. Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system. Invest. Ophthalmol. Vis. Sci. 50, 4205–4214. https://doi.org/10.1167/iovs.08-3214 (2009).
Zhou, B. et al. Mitochondrial quality control protects photoreceptors against oxidative stress in the H(2)O(2)-induced models of retinal degeneration diseases. Cell. Death Dis. 12, 413. https://doi.org/10.1038/s41419-021-03660-5 (2021).
Gu, X., Ma, Y., Liu, Y. & Wan, Q. Measurement of mitochondrial respiration in adherent cells by seahorse XF96 cell Mito stress test. STAR. Protoc. 2, 100245. https://doi.org/10.1016/j.xpro.2020.100245 (2021).
Wittig, I., Braun, H. P. & Schägger, H. Blue native PAGE. Nat. Protoc. 1, 418–428. https://doi.org/10.1038/nprot.2006.62 (2006).
Lin, C. S. et al. Mouse MtDNA mutant model of leber hereditary optic neuropathy. Proc. Natl. Acad. Sci. U S A. 109, 20065–20070. https://doi.org/10.1073/pnas.1217113109 (2012).
Wassmer, S. J. et al. XIAP protects retinal ganglion cells in the mutant ND4 mouse model of leber hereditary optic neuropathy. Invest. Ophthalmol. Vis. Sci. 61, 49. https://doi.org/10.1167/iovs.61.8.49 (2020).
Lambiri, D. W. & Levin, L. A. Modeling reactive oxygen species-induced axonal loss in leber hereditary optic neuropathy. Biomolecules 12 https://doi.org/10.3390/biom12101411 (2022).
Collins, J. A., Moots, R. J., Clegg, P. D. & Milner, P. I. Resveratrol and N-acetylcysteine influence redox balance in equine articular chondrocytes under acidic and very low oxygen conditions. Free Radic Biol. Med. 86, 57–64. https://doi.org/10.1016/j.freeradbiomed.2015.05.008 (2015).
Lardy, H. A. & Ferguson, S. M. Oxidative phosphorylation in mitochondria. Annu. Rev. Biochem. 38, 991–1034. https://doi.org/10.1146/annurev.bi.38.070169.005015 (1969).
Flood, D., Lee, E. S. & Taylor, C. T. Intracellular energy production and distribution in hypoxia. J. Biol. Chem. 299, 105103. https://doi.org/10.1016/j.jbc.2023.105103 (2023).
Hatefi, Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 54, 1015–1069. https://doi.org/10.1146/annurev.bi.54.070185.005055 (1985).
Senior, A. E. ATP synthesis by oxidative phosphorylation. Physiol. Rev. 68, 177–231. https://doi.org/10.1152/physrev.1988.68.1.177 (1988).
Dimauro, S. & Davidzon, G. Mitochondrial DNA and disease. Ann. Med. 37, 222–232. https://doi.org/10.1080/07853890510007368 (2005).
Fontana, G. A. & Gahlon, H. L. Mechanisms of replication and repair in mitochondrial DNA deletion formation. Nucleic Acids Res. 48, 11244–11258. https://doi.org/10.1093/nar/gkaa804 (2020).
Formosa, L. E. et al. Optic atrophy-associated TMEM126A is an assembly factor for the ND4-module of mitochondrial complex I. Proc. Natl. Acad. Sci. U. S. A. 118 https://doi.org/10.1073/pnas.2019665118 (2021).
Yu-Wai-Man, P. et al. Bilateral visual improvement with unilateral gene therapy injection for leber hereditary optic neuropathy. Sci. Transl. Med. 12 https://doi.org/10.1126/scitranslmed.aaz7423 (2020).
Newman, N. J. et al. Randomized trial of bilateral gene therapy injection for m.11778G > A MT-ND4 leber optic neuropathy. Brain 146, 1328–1341. https://doi.org/10.1093/brain/awac421 (2023).
Koilkonda, R. et al. LHON gene therapy vector prevents visual loss and optic neuropathy induced by G11778A mutant mitochondrial DNA: biodistribution and toxicology profile. Invest. Ophthalmol. Vis. Sci. 55, 7739–7753. https://doi.org/10.1167/iovs.14-15388 (2014).
Yu, H. et al. Mutant NADH dehydrogenase subunit 4 gene delivery to mitochondria by targeting sequence-modified adeno-associated virus induces visual loss and optic atrophy in mice. Mol. Vis. 18, 1668–1683 (2012).
Cwerman-Thibault, H. et al. Nuclear expression of mitochondrial ND4 leads to the protein assembling in complex I and prevents optic atrophy and visual loss. Mol. Ther. Methods Clin. Dev. 2, 15003. https://doi.org/10.1038/mtm.2015.3 (2015).
Yu, H. et al. Consequences of zygote injection and germline transfer of mutant human mitochondrial DNA in mice. Proc. Natl. Acad. Sci. U S A. 112, E5689–5698. https://doi.org/10.1073/pnas.1506129112 (2015).
Oca-Cossio, J., Kenyon, L., Hao, H. & Moraes, C. T. Limitations of allotopic expression of mitochondrial genes in mammalian cells. Genetics 165, 707–720. https://doi.org/10.1093/genetics/165.2.707 (2003).
Nieto-Panqueva, F., Rubalcava-Gracia, D. & Hamel, P. P. González-Halphen, D. The constraints of allotopic expression. Mitochondrion 73, 30–50. https://doi.org/10.1016/j.mito.2023.09.004 (2023).
Lam, B. L. et al. Leber hereditary optic neuropathy gene therapy: adverse events and visual acuity results of all patient groups. Am. J. Ophthalmol. 241, 262–271. https://doi.org/10.1016/j.ajo.2022.02.023 (2022).
Aguer, C. et al. Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS One. 6, e28536. https://doi.org/10.1371/journal.pone.0028536 (2011).
Elkalaf, M., Anděl, M. & Trnka, J. Low glucose but not galactose enhances oxidative mitochondrial metabolism in C2C12 myoblasts and myotubes. PLoS One. 8, e70772. https://doi.org/10.1371/journal.pone.0070772 (2013).
Acknowledgements
We thank Dr. Linying Zhou and Dr. Xi Lin (Electron Microscopy Lab of Public Technology Service Center, Fujian Medical University) for kindly providing technical assistance in electron microscopy.
Funding
This research received generous support from Joint Funds for the Innovation of Science and Technology, Fujian Province under grant No. 2024Y9336 and No. 2023Y9185. Additionally, it was made possible through the sponsorship of the Fujian Province Natural Science Foundation under grant No. 2022J01739 and the Fujian Provincial Health Technology Project under grant No. 2022GGA018.
Author information
Authors and Affiliations
Contributions
1. Lijun Fang: wrote the main manuscript and performed research. 2. Kangyue Fu, Mengyu Yang, Yiwen Xu and Ezeugwu Sussan Ukamaka: analyzed data and performed research. 3. Dianbo Qu: wrote the manuscript and analyzed data. 4. Tianwen Huang and Jianzhang Hu: wrote the main manuscript text, performed research and designed research. All the authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fang, L., Fu, K., Yang, M. et al. Oxidative stress imbalance and cellular damage mediated by the ND4 G11778A mutation. Sci Rep (2026). https://doi.org/10.1038/s41598-026-40061-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-40061-0