Abstract
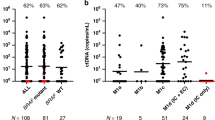
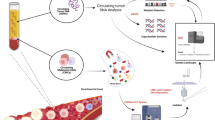
Progression occurs in more than 10% of melanoma patients initially diagnosed with local disease, emphasizing the need for improved risk stratification even in early stages. In this study, we used an enrichment method based on recombinant Plasmodium falciparum VAR2CSA protein to enable the capture of rare circulating tumor cells (CTCs) from a single blood draw in early-stage (AJCC I-II) melanoma patients. CTCs were subsequently identified using fluorescent antibodies targeting tumor initiating and melanoma specific cell markers. In parallel, we investigated circulating tumor DNA (ctDNA) in these patients. Among 92 early-stage melanoma patients, CTCs were detected in 21 patients (22.8%) at time of initial diagnosis. The presence of CTCs was significantly associated with an unfavorable clinical outcome, such as disease progression or death related to melanoma disease, with a median observation time of 30 months (IQR 24.0–36.0) (p = 0.043). Furthermore, when combining CTC with ctDNA detection, a highly significant correlation with disease progression was observed (p = 0.014) underlining the role of early tumor cell dissemination in melanoma disease. These findings suggest that CTC assessment, particularly when combined with ctDNA analysis, may represent a valuable biomarker to facilitate risk stratification of early-stage melanoma patients to improve personalized treatment approaches.
Similar content being viewed by others
Data availability
The sequence data generated and analyzed during the current study is available in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB98726 (https://www.ebi.ac.uk/ena/browser/view/PRJEB98726).
References
Lyth, J. Conditional recurrence-free survival in patients with primary stage I-II cutaneous malignant melanoma–a population-based study. Melanoma Res. 28(6), 637–640 (2018).
Garbe, C. et al. Prognosis of patients with primary melanoma stage I and II according to american joint committee on cancer version 8 validated in two independent cohorts: implications for adjuvant treatment. J. Clin. Oncol. 40(32), 3741–3749 (2022).
Hou, J.-M. et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 30, 525–532 (2012).
Lawrence, R., Watters, M., Davies, C. R., Pantel, K. & Lu, Y.-J. Circulating tumour cells for early detection of clinically relevant cancer. Nat. Rev. Clin. Oncol. 20, 487–500 (2023).
Kamińska, P. et al. Liquid biopsy in melanoma: Significance in diagnostics, prediction and treatment monitoring. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22189714 (2021).
Boyer, M. et al. Clinical relevance of liquid biopsy in melanoma and merkel cell carcinoma. Cancers https://doi.org/10.3390/cancers12040960 (2020).
Slusher, N., Jones, N. & Nonaka, T. Liquid biopsy for diagnostic and prognostic evaluation of melanoma. Front. Cell Develop. Biol. https://doi.org/10.3389/fcell.2024.1420360 (2024).
Kiniwa, Y. et al. Usefulness of monitoring circulating tumor cells as a therapeutic biomarker in melanoma with BRAF mutation. BMC Cancer 21, 287 (2021).
Roland, C. L. et al. Detection of circulating melanoma cells in the blood of melanoma patients. Melanoma Res. 25, 335–341 (2015).
Edgar, R. H. et al. Predicting metastasis in melanoma by enumerating circulating tumor cells using photoacoustic flow cytometry. Lasers Surg. Med. 53, 578–586 (2021).
Kang, Y. T. et al. Isolation of circulating tumor cells to diagnose melanoma and evaluate the efficacy of surgical resection using melanoma-specific microsystem. Adv. Nanobiomed Res. 2(8), 2100083 (2022).
Gray, E. S. et al. Circulating melanoma cell subpopulations: Their heterogeneity and differential responses to treatment. J. Invest. Dermatol. 135, 2040–2048 (2015).
Vidal-Calvo, E. E. et al. Tumor-agnostic cancer therapy using antibodies targeting oncofetal chondroitin sulfate. Nat. Commun. 15, 7553 (2024).
Salanti, A. et al. Targeting human cancer by a glycosaminoglycan binding malaria protein. Cancer Cell 28, 500–514 (2015).
Clausen, T. M. et al. Oncofetal chondroitin sulfate glycosaminoglycans are key players in integrin signaling and tumor cell motility. Mol. Cancer Res. 14, 1288–1299 (2016).
Løppke, C. et al. Multi-cancer detection of circulating tumor cells by targeting oncofetal chondroitin sulfate. NPJ Precis. Oncol. 9, 144 (2025).
Kupas, V. et al. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. J. Investig. Dermatol. 131, 944–955 (2011).
Freeman, J. B., Gray, E. S., Millward, M., Pearce, R. & Ziman, M. Evaluation of a multi-marker immunomagnetic enrichment assay for the quantification of circulating melanoma cells. J. Transl. Med. 10, 192 (2012).
Cayrefourcq, L. et al. S100-EPISPOT: A new tool to detect viable circulating melanoma cells. Cells 8(7), 755 (2019).
Lucci, A. et al. Circulating tumor cells and early relapse in node-positive melanoma. Clin. Cancer Res. 26, 1886–1895 (2020).
Gorges, K. et al. Intra-patient heterogeneity of circulating tumor cells and circulating tumor DNA in Blood of melanoma patients. Cancers 11(11), 1685 (2019).
Guetter, S. et al. MCSP+ metastasis founder cells activate immunosuppression early in human melanoma metastatic colonization. Nat. Cancer https://doi.org/10.1038/s43018-025-00963-w (2025).
Schroeder, C. et al. Tumour-informed liquid biopsies to monitor advanced melanoma patients under immune checkpoint inhibition. Nat. Commun. 15, 8750 (2024).
Gray, E. S. et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget 6, 42008–42018 (2015).
Aung, K. L. et al. Analytical validation of BRAF mutation testing from circulating free DNA using the amplification refractory mutation testing system. J. Mol. Diagn. 16, 343–349 (2014).
Forschner, A. et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma - results of a prospective biomarker study. J. Immunother Cancer 7, 180 (2019).
Grzywa, T. M., Paskal, W. & Włodarski, P. K. Intratumor and Intertumor Heterogeneity in Melanoma. Transl. Oncol. 10, 956–975 (2017).
Agerbæk, M. Ø. et al. The VAR2CSA malaria protein efficiently retrieves circulating tumor cells in an EpCAM-independent manner. Nat. Commun. 9, 3279 (2018).
Fourdrain, L. et al. Detection of circulating tumor cells is achieved by flow cytometry in melanoma patients. Cytometry B Clin. Cytom. 108, 312–319 (2025).
Hoshimoto, S. et al. Association between circulating tumor cells and prognosis in patients with stage III melanoma with sentinel lymph node metastasis in a phase III international multicenter trial. J. Clin. Oncol. 30, 3819–3826 (2012).
Venturi, F., Magnaterra, E., Scotti, B., Ferracin, M. & Dika, E. Predictive Factors for sentinel lymph node positivity in melanoma patients: The role of liquid biopsy, microrna and gene expression profile panels. Cancers https://doi.org/10.3390/cancers17081281 (2025).
Han, M. et al. In vivo lymphatic circulating tumor cells and progression of metastatic disease. Cancers (Basel) 12, 1–18 (2020).
Bettegowda, C. et al. Detection of circulating tumor DNA in early-and late-stage human malignancies. Sci. Transl. Med. 6(224), 224ra24 (2014).
Gershenwald, J. E. et al. Melanoma staging: Evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 67, 472–492 (2014).
Sand, N. T. et al. Optimization of rVAR2-based isolation of cancer cells in blood for building a robust assay for clinical detection of circulating tumor cells. Int. J. Mol. Sci. 21(7), 2401 (2020).
Zakeri, B. et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. https://doi.org/10.1073/pnas.1115485109
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Ellard, S. et al. ACGS Best Practice Guidelines for Variant Classification in Rare Disease 2020. bioRxiv (2020).
Acknowledgements
This research was supported, in part, by VAR2 Pharmaceuticals. The funders had no role in data collection and analysis, decision to publish and original draft preparation. We thank the Department of Dermatology (Ordensklinikum Linz Elisabethinen, Austria), the Laboratory for Molecular and Genetic Diagnostics (Ordensklinikum Linz Barmherzige Schwestern, Austria) and the Department of Immunology and Microbiology (University of Copenhagen, Denmark) for the cooperation and providing their clinical, moleculargenetic and biochemical expertise.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The conducted work was funded by the Department of Dermatology (Ordensklinikum Linz Elisabethinen) and the Laboratory of Molecular Genetic Diagnostics (Ordensklinikum Linz Barmherzige Schwestern) and their research funds.
Author information
Authors and Affiliations
Contributions
J.S and A.S. are co-corresponding authors. J.S. and M.R. designed and conducted the experiments, contributed to data analysis and interpretation and wrote the manuscript; L.L. contributed to data analysis and writing the manuscript; L.S. and V.B. assisted with the analysis; E.S. contributed to patient data collection; K.P., V.P. and H.K. contributed to sample and patient data collection. T.G.T. and P.S. helped with the conceptualization. G.W., A.S., M. Ø.A. and N.S. provided resources and contributed to the conceptualization, validation and editing of the manuscript. J.S., M.R., A.S., M.Ø.A., N.S. did a critical revision of the manuscript for important intellectual content: Final approval of completed version of manuscript: All authors.
Corresponding authors
Ethics declarations
Competing interests
H.K. is consultant for MSD, Bristol Myers Squibb and Pierre Fabre. The aforementioned functions are unrelated to the research presented in this manuscript. The technology to diagnose cancer through rVAR2 is owned by VarCT Diagnostics through a license from VAR2Pharmaceuticals. A.S., T.G.T., M.Ø.A. and P.S. are cofounders of VAR2Pharmaceuticals. The other authors declare no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sunzenauer, J., Rammer, M., Stöckl, L. et al. Oncofetal chondroitin sulfate positive circulating tumor cells as prognostic biomarkers in early-stage melanoma. Sci Rep (2026). https://doi.org/10.1038/s41598-026-40072-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-40072-x