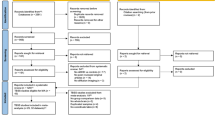
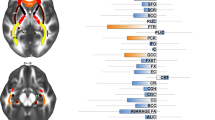
Abstract
Attention deficit/hyperactivity disorder (ADHD) is typically associated with working memory deficits, which are thought to arise from impaired attentional control. Previous research has highlighted abnormalities in alpha oscillations (8–12 Hz) during working memory tasks in children with ADHD, particularly attenuated event-related alpha power decreases (alpha ERD). However, the structural underpinnings of these oscillatory dynamics remain unclear. This study aimed to investigate the relationship between white matter microstructure and alpha modulation during a spatial working memory task in children with ADHD and typically developing (TD) controls. EEG and diffusion tensor imaging (DTI) data were analyzed from 115 children (ADHD n = 72; TD n = 43). We focused on three white matter tracts: the optic radiation (OR), anterior thalamic radiation (ATR), and the second branch of the superior longitudinal fasciculus (SLF2). DTI analyses revealed increased mean diffusivity in the ATR and SLF2 in ADHD, indicating reduced white matter integrity. Importantly, ATR microstructure significantly predicted alpha ERD, suggesting a key role for anterior thalamic pathways in modulating neural oscillations during working memory encoding. In contrast, SLF2 and OR microstructure did not significantly influence alpha modulation. These findings support a thalamus-mediated model of alpha modulation, where disruptions in anterior thalamic microstructural integrity contribute to attentional impairments in ADHD. Understanding these structural-functional relationships may inform targeted interventions aimed at improving executive function in this population.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data is not publicly available due to privacy or ethical restrictions.
References
Castellanos, F. X. & Tannock, R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat. Rev. Neurosci. 3, 617–628 (2002).
Lenartowicz, A., Mazaheri, A., Jensen, O. & Loo, S. K. Aberrant modulation of brain oscillatory activity and attentional impairment in attention-deficit/hyperactivity disorder. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging. 3, 19–29 (2018).
Lenartowicz, A. et al. Electroencephalography correlates of spatial working memory deficits in attention-deficit/hyperactivity disorder: vigilance, encoding, and maintenance. J. Neurosci. 34, 1171–1182 (2014).
Lenartowicz, A. et al. Alpha modulation during working memory encoding predicts neurocognitive impairment in ADHD. J. Child. Psychol. Psychiatr Jcpp. 13042 https://doi.org/10.1111/jcpp.13042 (2019).
Mazaheri, A. et al. Differential oscillatory electroencephalogram between attention-deficit/hyperactivity disorder subtypes and typically developing adolescents. Biol. Psychiatry. 76, 422–429 (2014).
Michelini, G., Salmastyan, G., Vera, J. D. & Lenartowicz, A. Event-related brain oscillations in attention-deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. Int. J. Psychophysiol. 174, 29–42 (2022).
Foxe, J. J. & Snyder, A. C. The role of Alpha-Band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychology 2, 154 (2011).
Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16, 606–617 (2012).
Klimesch, W., Fellinger, R. & Freunberger, R. Alpha oscillations and early stages of visual encoding. Front. Psychol. 2, 1–11 (2011).
Mathewson, K. E. et al. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Front Psychology 2, 99 (2011).
Lenartowicz, A. et al. Alpha desynchronization and frontoparietal connectivity during spatial working memory encoding deficits in ADHD: A simultaneous EEG-fMRI study. NeuroImage: Clin. 11, 210–223 (2016).
Zumer, J. M., Scheeringa, R., Schoffelen, J. M., Norris, D. G. & Jensen, O. Occipital alpha activity during stimulus processing gates the information flow to object-selective cortex. PLoS Biol. 12, e1001965 (2014).
Nunez, P. L., Srinivasan, R. & Fields, R. D. EEG functional connectivity, axon delays and white matter disease. Clin. Neurophysiol. 126, 110–120 (2015).
Nunez, P. L. & Srinivasan, R. Neocortical dynamics due to axon propagation delays in cortico-cortical fibers: EEG traveling and standing waves with implications for top-down influences on local networks and white matter disease. Brain Res. 1542, 138–166 (2014).
Lenartowicz, A., Coleman, S. C., Zink, N. & Mullinger, K. J. The multidimensional relationship between alpha oscillations and cognition. Imaging Neurosci. (Camb) 3, 96 (2025).
Hughes, S. W. & Crunelli, V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist 11, 357–372 (2005).
Saalmann, Y. B. & Kastner, S. Cognitive and perceptual functions of the visual thalamus. Neuron 71, 209–223 (2011).
Marshall, T. R., Bergmann, T. O. & Jensen, O. Frontoparietal structural connectivity mediates the top-down control of neuronal synchronization associated with selective attention. PLoS Biol. 13, e1002272 (2015).
van Ewijk, H. et al. Different mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. J. Am. Acad. Child. Adolesc. Psychiatry. 53, 790–799e3 (2014).
Wu, Z. M. et al. White matter microstructural alterations in children with ADHD: categorical and dimensional perspectives. Neuropsychopharmacol 42, 572–580 (2017).
Lawrence, K. E. et al. White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings. J. Am. Acad. Child. Adolesc. Psychiatry. 52, 431–440e4 (2013).
Wu, Z. M. et al. Altered brain white matter microstructural asymmetry in children with ADHD. Psychiatry Res. 285, 112817 (2020).
Sarubbo, S. et al. The course and the anatomo-functional relationships of the optic radiation: a combined study with ‘post mortem’ dissections and ‘in vivo’ direct electrical mapping. J. Anat. 226, 47–59 (2015).
Caffarra, S. et al. Development of the alpha rhythm is linked to visual white matter pathways and visual detection performance. J. Neurosci. 44, e0684232023 (2024).
Cowan, F. M. & de Vries, L. The internal capsule in neonatal imaging. Seminars Fetal Neonatal Med. 10, 461–474 (2005).
Wakana, S. et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 36, 630–644 (2007).
De Thiebaut, M. et al. A lateralized brain network for visuospatial attention. Nat. Neurosci. 14, 1245–1246 (2011).
D’Andrea, A. et al. Alpha and alpha-beta phase synchronization mediate the recruitment of the visuospatial attention network through the superior longitudinal fasciculus. NeuroImage 188, 722–732 (2019).
Mazzetti, C. et al. Dorsal-to‐ventral imbalance in the superior longitudinal fasciculus mediates methylphenidate’s effect on beta oscillations in ADHD. Psychophysiology 59, e14008 (2022).
Bilder, R. M. et al. Cognitive effects of stimulant, guanfacine, and combined treatment in child and adolescent attention-deficit/hyperactivity disorder. J. Am. Acad. Child. Adolesc. Psychiatry. 55, 667–673 (2016).
Loo, S. K. et al. Effects of d-Methylphenidate, Guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J. Am. Acad. Child. Adolesc. Psychiatry. 55, 674–682e1 (2016).
McCracken, J. T. et al. Combined stimulant and guanfacine administration in attention-deficit/hyperactivity disorder: A controlled, comparative study. J. Am. Acad. Child. Adolesc. Psychiatry. 55, 657–666e1 (2016).
Kaufman, J. et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child. Adolesc. Psychiatry. 36, 980–988 (1997).
Swanson, J. M. et al. Categorical and dimensional definitions and evaluations of symptoms of ADHD: history of the SNAP and the SWAN rating scales. Int. J. Educ. Psychol. Assess. 10, 51–70 (2012).
Glahn, D. C. et al. Maintenance and Manipulation in Spatial Working Memory: Dissociations in the Prefrontal Cortex. NeuroImage 17, 201–213 (2002).
Sternberg, S. High-speed scanning in human memory. Science 153, 652–654 (1966).
Klem, G. H., Lüders, H. O., Jasper, H. H. & Elger, C. The ten-twenty electrode system of the international federation. The international federation of clinical neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 52, 3–6 (1999).
Delorme, A. & Makeig, S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 134, 9–21 (2004).
Jones, D. K. et al. Isotropic resolution diffusion tensor imaging with whole brain acquisition in a clinically acceptable time. Hum. Brain. Mapp. 15, 216–230 (2002).
Alexander, A. L., Lee, J. E., Lazar, M. & Field, A. S. Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329 (2007).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, S208–S219 (2004).
Schilling, K. G. et al. Synthesized b0 for diffusion distortion correction (Synb0-DisCo). Magn. Reson. Imaging. 64, 62–70 (2019).
Andersson, J. L. R., Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 20, 870–888 (2003).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078 (2016).
Andersson, J. L. R., Graham, M. S., Zsoldos, E. & Sotiropoulos, S. N. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage 141, 556–572 (2016).
Andersson, J. L. R., Graham, M. S., Drobnjak, I., Zhang, H. & Campbell, J. Susceptibility-induced distortion that varies due to motion: correction in diffusion MR without acquiring additional data. NeuroImage 171, 277–295 (2018).
Bastiani, M. et al. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. NeuroImage 184, 801–812 (2019).
Hernández, M. et al. Accelerating fibre orientation estimation from diffusion weighted magnetic resonance imaging using GPUs. PLOS ONE. 8, e61892 (2013).
Yendiki, A. et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform 5, 23 (2011).
Maffei, C. et al. Using diffusion MRI data acquired with ultra-high gradient strength to improve tractography in routine-quality data. NeuroImage 245, 118706 (2021).
Yendiki, A., Koldewyn, K., Kakunoori, S., Kanwisher, N. & Fischl, B. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage 88, 79–90 (2014).
Cohen, J., Cohen, P., West, S. G., & Aiken, L. S. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences (L. Erlbaum Associates, 2003).
Little, T. D., Bovaird, J. A. & Widaman, K. F. On the merits of orthogonalizing powered and product terms: implications for modeling interactions among latent variables. Struct. Equ. Model. 13, 497–519 (2006).
Rosseel, Y. Lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36 (2012).
Aoki, Y., Cortese, S. & Castellanos, F. X. Research review: diffusion tensor imaging studies of attention-deficit/hyperactivity disorder: meta‐analyses and reflections on head motion. Child. Psychol. Psychiatry. 59, 193–202 (2018).
O’Connor, D. H., Fukui, M. M., Pinsk, M. A. & Kastner, S. Attention modulates responses in the human lateral geniculate nucleus. Nat. Neurosci. 5, 1203–1209 (2002).
Halassa, M. M. & Kastner, S. Thalamic functions in distributed cognitive control. Nat. Neurosci. 20, 1669–1679 (2017).
Saalmann, Y. B. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front Syst. Neurosci 8, 83 (2014).
Saalmann, Y. B., Pinsk, M. A., Wang, L., Li, X. & Kastner, S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science 337, 753–756 (2012).
Keehn, B., Westerfield, M., Müller, R. A., & Townsend, J. Autism, attention, and alpha oscillations: an electrophysiological study of attentional capture. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging. 2, 528–536 (2017).
Bottari, D. et al. Sight restoration after congenital blindness does not reinstate alpha oscillatory activity in humans. Sci. Rep. 6, 24683 (2016).
Janelle, F., Iorio-Morin, C., D’amour, S. & Fortin, D. Superior longitudinal fasciculus: A review of the anatomical descriptions with functional correlates. Front Neurol 13, 794618 (2022).
Acknowledgements
This work used computational and storage services associated with the Hoffman2 Shared Cluster provided by UCLA Office of Advanced Research Computing’s Research Technology Group.
Funding
This work was supported by the National Institute of Mental Health (NIMH) grant numbers: R01MH116268 (Lenartowicz/Loo) and 5P50MH077248 (McCracken).
Author information
Authors and Affiliations
Contributions
Joel P. Diaz-Fong: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft; James McGough: Supervision; James T. McCracken: Funding acquisition, Investigation; Sandra K. Loo: Data curation, Funding acquisition, Supervision, Writing – review & editing; Agatha Lenartowicz: Data curation, Formal analysis, Funding acquisition, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Diaz-Fong, J.P., McGough, J., McCracken, J.T. et al. Structural integrity of the anterior thalamic radiation predicts alpha oscillations and inattention during visual encoding. Sci Rep (2026). https://doi.org/10.1038/s41598-026-40086-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-40086-5